Bariatric surgery in adolescents: a prospective randomized controlled trial comparing laparoscopic gastric banding to combined lifestyle interventions in adolescents with severe obesity (BASIC trial)
- PMID: 30691442
- PMCID: PMC6350363
- DOI: 10.1186/s12887-019-1395-9
Bariatric surgery in adolescents: a prospective randomized controlled trial comparing laparoscopic gastric banding to combined lifestyle interventions in adolescents with severe obesity (BASIC trial)
Abstract
Background: Obesity in children and adolescents is an increasing problem associated with multiple co-morbidities including metabolic and endocrine changes, cardiovascular abnormalities, and impaired quality of life. Combined lifestyle interventions are the current standard treatment for severe obesity in children. However, the medium- and long-term results of these interventions are relatively poor. Bariatric surgery shows substantial weight loss and health improvement in adults and retrospective studies in adolescents show similar outcomes. However, well-designed prospective studies in this young age group are rare. Our objectives are to determine whether combining surgery with lifestyle interventions in severely obese adolescents leads to a significant additional weight reduction compared to lifestyle interventions solely, and to assess its effect on obesity-associated co-morbidities in a prospective randomized controlled setting.
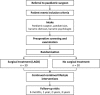
Methods: Patients aged 14-16 years with sex- and age-adjusted BMI > 40 kg/m2 (or > 35 kg/m2 with comorbidity) and failure to achieve weight reduction > 5% during at least one year of combined lifestyle interventions are included in this trial. Randomization determines whether laparoscopic adjustable gastric banding will be added to combined lifestyle intervention throughout the trial period. Sixty children will be included in this trial. Follow-up visits are planned at 6 months, 1,2 and 3 years. Primary endpoints are percentage of total weight loss, and change of BMI. Secondary endpoints include body composition, pubertal development, metabolic and endocrine changes, inflammatory status, cardiovascular abnormalities, non-alcoholic steatohepatitis, quality of life and changes in behaviour.
Discussion: This randomized controlled trial is designed to provide important information about the safety and efficacy of laparoscopic adjustable gastric banding treatment in severely obese adolescents with unsuccessful combined lifestyle interventions. The reversibility of this surgical procedure forms a strong argument to decide for gastric banding over other surgical procedures, since bariatric surgery in adolescents is still in its infancy.
Trial registration: The BASIC trial is registered in the register of ClinicalTrials.gov since July 2010, Identifier: NCT01172899.
Keywords: Adolescents; Bariatric surgery; Safety; Severe obesity; Weight loss.
Conflict of interest statement
Ethics approval and consent to participate
The study is conducted in accordance to the standards of Good Clinical Practice, in agreement with the Declaration of Helsinki and with Dutch law in general and with the Act Medical Research Involving Human Subjects in particular. This study was approved by the medical ethics committee of Maastricht University Medical Centre (NL26279.068.09 / METC 09–2-024). A written informed consent was signed by all patients included in this trial and their parents / legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization: Global status report on noncommunicable diseases 2010. In.: World Health Organization; 2011: 176.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical