Selective targeting of NAMPT by KPT-9274 in acute myeloid leukemia
- PMID: 30692102
- PMCID: PMC6373756
- DOI: 10.1182/bloodadvances.2018024182
Selective targeting of NAMPT by KPT-9274 in acute myeloid leukemia
Abstract
Treatment options for acute myeloid leukemia (AML) remain extremely limited and associated with significant toxicity. Nicotinamide phosphoribosyltransferase (NAMPT) is involved in the generation of NAD+ and a potential therapeutic target in AML. We evaluated the effect of KPT-9274, a p21-activated kinase 4/NAMPT inhibitor that possesses a unique NAMPT-binding profile based on in silico modeling compared with earlier compounds pursued against this target. KPT-9274 elicited loss of mitochondrial respiration and glycolysis and induced apoptosis in AML subtypes independent of mutations and genomic abnormalities. These actions occurred mainly through the depletion of NAD+, whereas genetic knockdown of p21-activated kinase 4 did not induce cytotoxicity in AML cell lines or influence the cytotoxic effect of KPT-9274. KPT-9274 exposure reduced colony formation, increased blast differentiation, and diminished the frequency of leukemia-initiating cells from primary AML samples; KPT-9274 was minimally cytotoxic toward normal hematopoietic or immune cells. In addition, KPT-9274 improved overall survival in vivo in 2 different mouse models of AML and reduced tumor development in a patient-derived xenograft model of AML. Overall, KPT-9274 exhibited broad preclinical activity across a variety of AML subtypes and warrants further investigation as a potential therapeutic agent for AML.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosures: E.B. and W.S. are employees of, and have financial interests in, Karyopharm Therapeutics Inc. J.S.B. has performed consulting for AbbVie, AstraZeneca, and Kite Pharma. V.K.P. performed consulting for Orbus Therapeutics and SK Biosciences. The remaining authors declare no competing financial interests.
Figures


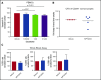
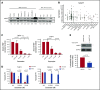




References
-
- Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894-1907. - PubMed
-
- Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. - PubMed
-
- Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;129(26):3403-3406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

