Staphylococcus aureus Biofilm-Conditioned Medium Impairs Macrophage-Mediated Antibiofilm Immune Response by Upregulating KLF2 Expression
- PMID: 30692179
- PMCID: PMC6434135
- DOI: 10.1128/IAI.00643-18
Staphylococcus aureus Biofilm-Conditioned Medium Impairs Macrophage-Mediated Antibiofilm Immune Response by Upregulating KLF2 Expression
Abstract
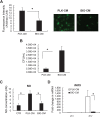
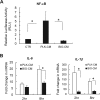
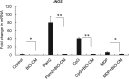
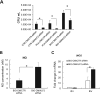
Staphylococcus aureus infections associated with the formation of biofilms on medical implants or host tissue play a critical role in the persistence of chronic infections. One critical mechanism of biofilm infection that leads to persistent infection lies in the capacity of biofilms to evade the macrophage-mediated innate immune response. It is now increasingly apparent that microorganisms exploit the negative regulatory mechanisms of the pattern recognition receptor (PRR)-mediated inflammatory response to subvert host cell functions by using various virulence factors. However, the detailed molecular mechanism, along with the identity of a target molecule, underlying the evasion of the macrophage-mediated innate immune response against S. aureus infection associated with biofilm formation remains to be elucidated. Here, using an in vitro culture model of murine macrophage-like RAW 264.7 cells, we demonstrate that S. aureus biofilm-conditioned medium significantly attenuated the capacity for macrophage bactericidal and proinflammatory responses. Importantly, the responses were associated with attenuated activation of NF-κB and increased expression of Kruppel-like factor 2 (KLF2) in RAW 264.7 cells. Small interfering RNA (siRNA)-mediated silencing of KLF2 in RAW 264.7 cells could restore the activation of NF-κB toward the bactericidal activity and generation of proinflammatory cytokines in the presence of S. aureus biofilm-conditioned medium. Collectively, our results suggest that factors secreted from S. aureus biofilms might exploit the KLF2-dependent negative regulatory mechanism to subvert macrophage-mediated innate immune defense against S. aureus biofilms.
Keywords: KLF2; S. aureus biofilm; macrophages.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E. 2005. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 165:1756–1761. doi:10.1001/archinte.165.15.1756. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

