Intracellular Degradation of Helicobacter pylori VacA Toxin as a Determinant of Gastric Epithelial Cell Viability
- PMID: 30692181
- PMCID: PMC6434121
- DOI: 10.1128/IAI.00783-18
Intracellular Degradation of Helicobacter pylori VacA Toxin as a Determinant of Gastric Epithelial Cell Viability
Abstract
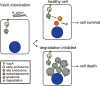
Helicobacter pylori VacA is a secreted pore-forming toxin that induces cell vacuolation in vitro and contributes to the pathogenesis of gastric cancer and peptic ulcer disease. We observed that purified VacA has relatively little effect on the viability of AGS gastric epithelial cells, but the presence of exogenous weak bases such as ammonium chloride (NH4Cl) enhances the susceptibility of these cells to VacA-induced vacuolation and cell death. Therefore, we tested the hypothesis that NH4Cl augments VacA toxicity by altering the intracellular trafficking of VacA or inhibiting intracellular VacA degradation. We observed VacA colocalization with LAMP1- and LC3-positive vesicles in both the presence and absence of NH4Cl, indicating that NH4Cl does not alter VacA trafficking to lysosomes or autophagosomes. Conversely, we found that supplemental NH4Cl significantly increases the intracellular stability of VacA. By conducting experiments using chemical inhibitors, stable ATG5 knockdown cell lines, and ATG16L1 knockout cells (generated using CRISPR/Cas9), we show that VacA degradation is independent of autophagy and proteasome activity but dependent on lysosomal acidification. We conclude that weak bases like ammonia, potentially generated during H. pylori infection by urease and other enzymes, enhance VacA toxicity by inhibiting toxin degradation.
Keywords: Helicobacter pylori; VacA; cell death; cell survival; pore-forming toxins.
Copyright © 2019 American Society for Microbiology.
Figures





Similar articles
-
Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin.Cancer Res. 2003 Mar 1;63(5):951-7. Cancer Res. 2003. PMID: 12615708
-
Instrumental Role of Helicobacter pylori γ-Glutamyl Transpeptidase in VacA-Dependent Vacuolation in Gastric Epithelial Cells.PLoS One. 2015 Jun 25;10(6):e0131460. doi: 10.1371/journal.pone.0131460. eCollection 2015. PLoS One. 2015. PMID: 26111186 Free PMC article.
-
VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori infection.Sci Rep. 2019 Jan 10;9(1):38. doi: 10.1038/s41598-018-37095-4. Sci Rep. 2019. PMID: 30631092 Free PMC article.
-
An Overview of Helicobacter pylori VacA Toxin Biology.Toxins (Basel). 2016 Jun 3;8(6):173. doi: 10.3390/toxins8060173. Toxins (Basel). 2016. PMID: 27271669 Free PMC article. Review.
-
Vacuolating cytotoxin A (VacA) - A multi-talented pore-forming toxin from Helicobacter pylori.Toxicon. 2016 Aug;118:27-35. doi: 10.1016/j.toxicon.2016.04.037. Epub 2016 Apr 20. Toxicon. 2016. PMID: 27105670 Review.
Cited by
-
Restoration of mitochondrial structure and function within Helicobacter pylori VacA intoxicated cells.Adv Microbiol. 2023 Aug;13(8):399-419. doi: 10.4236/aim.2023.138026. Adv Microbiol. 2023. PMID: 37654621 Free PMC article.
-
Taurine modulates host cell responses to Helicobacter pylori VacA toxin.Infect Immun. 2024 Aug 13;92(8):e0022424. doi: 10.1128/iai.00224-24. Epub 2024 Jul 8. Infect Immun. 2024. PMID: 38975764 Free PMC article.
-
Gut Microbiome Alteration after Reboxetine Administration in Type-1 Diabetic Rats.Microorganisms. 2021 Sep 14;9(9):1948. doi: 10.3390/microorganisms9091948. Microorganisms. 2021. PMID: 34576843 Free PMC article.
-
AUF1-mediated inhibition of autophagic lysosomal degradation contributes to CagA stability and Helicobacter pylori-induced inflammation.Gut Microbes. 2024 Jan-Dec;16(1):2382766. doi: 10.1080/19490976.2024.2382766. Epub 2024 Jul 28. Gut Microbes. 2024. PMID: 39068523 Free PMC article.
-
The crosstalk between bacteria and host autophagy: host defense or bacteria offense.J Microbiol. 2022 May;60(5):451-460. doi: 10.1007/s12275-022-2009-z. Epub 2022 Apr 29. J Microbiol. 2022. PMID: 35486304 Review.
References
-
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. 2017. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153:420–429. doi:10.1053/j.gastro.2017.04.022. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

