A ZNRF3-dependent Wnt/β-catenin signaling gradient is required for adrenal homeostasis
- PMID: 30692207
- PMCID: PMC6362817
- DOI: 10.1101/gad.317412.118
A ZNRF3-dependent Wnt/β-catenin signaling gradient is required for adrenal homeostasis
Abstract
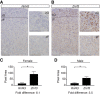
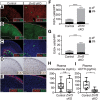
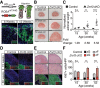
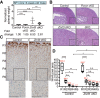
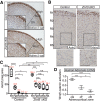
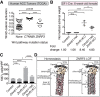
Spatiotemporal control of Wnt signaling is essential for the development and homeostasis of many tissues. The transmembrane E3 ubiquitin ligases ZNRF3 (zinc and ring finger 3) and RNF43 (ring finger protein 43) antagonize Wnt signaling by promoting degradation of frizzled receptors. ZNRF3 and RNF43 are frequently inactivated in human cancer, but the molecular and therapeutic implications remain unclear. Here, we demonstrate that adrenocortical-specific loss of ZNRF3, but not RNF43, results in adrenal hyperplasia that depends on Porcupine-mediated Wnt ligand secretion. Furthermore, we discovered a Wnt/β-catenin signaling gradient in the adrenal cortex that is disrupted upon loss of ZNRF3. Unlike β-catenin gain-of-function models, which induce high Wnt/β-catenin activation and expansion of the peripheral cortex, ZNRF3 loss triggers activation of moderate-level Wnt/β-catenin signaling that drives proliferative expansion of only the histologically and functionally distinct inner cortex. Genetically reducing β-catenin dosage significantly reverses the ZNRF3-deficient phenotype. Thus, homeostatic maintenance of the adrenal cortex is dependent on varying levels of Wnt/β-catenin activation, which is regulated by ZNRF3.
Keywords: Wnt signaling; adrenal zonation; mouse models; organ maintenance; proliferation.
© 2019 Basham et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrancois-Martinez AM, et al. 2010. Constitutive β-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 19: 1561–1576. 10.1093/hmg/ddq029 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
