Synthetic Switches and Regulatory Circuits in Plants
- PMID: 30692218
- PMCID: PMC6393786
- DOI: 10.1104/pp.18.01362
Synthetic Switches and Regulatory Circuits in Plants
Abstract
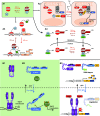
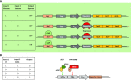
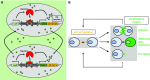
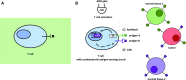
Synthetic biology is an established but ever-growing interdisciplinary field of research currently revolutionizing biomedicine studies and the biotech industry. The engineering of synthetic circuitry in bacterial, yeast, and animal systems prompted considerable advances for the understanding and manipulation of genetic and metabolic networks; however, their implementation in the plant field lags behind. Here, we review theoretical-experimental approaches to the engineering of synthetic chemical- and light-regulated (optogenetic) switches for the targeted interrogation and control of cellular processes, including existing applications in the plant field. We highlight the strategies for the modular assembly of genetic parts into synthetic circuits of different complexity, ranging from Boolean logic gates and oscillatory devices up to semi- and fully synthetic open- and closed-loop molecular and cellular circuits. Finally, we explore potential applications of these approaches for the engineering of novel functionalities in plants, including understanding complex signaling networks, improving crop productivity, and the production of biopharmaceuticals.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures







References
-
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 - PubMed
-
- Antunes MS, Ha SB, Tewari-Singh N, Morey KJ, Trofka AM, Kugrens P, Deyholos M, Medford JI (2006) A synthetic de-greening gene circuit provides a reporting system that is remotely detectable and has a re-set capacity. Plant Biotechnol J 4: 605–622 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

