Discrete Structure of the Brain Rhythms
- PMID: 30692564
- PMCID: PMC6349927
- DOI: 10.1038/s41598-018-37196-0
Discrete Structure of the Brain Rhythms
Abstract
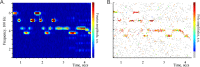
Neuronal activity in the brain generates synchronous oscillations of the Local Field Potential (LFP). The traditional analyses of the LFPs are based on decomposing the signal into simpler components, such as sinusoidal harmonics. However, a common drawback of such methods is that the decomposition primitives are usually presumed from the onset, which may bias our understanding of the signal's structure. Here, we introduce an alternative approach that allows an impartial, high resolution, hands-off decomposition of the brain waves into a small number of discrete, frequency-modulated oscillatory processes, which we call oscillons. In particular, we demonstrate that mouse hippocampal LFP contain a single oscillon that occupies the θ-frequency band and a couple of γ-oscillons that correspond, respectively, to slow and fast γ-waves. Since the oscillons were identified empirically, they may represent the actual, physical structure of synchronous oscillations in neuronal ensembles, whereas Fourier-defined "brain waves" are nothing but poorly resolved oscillons.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Boashash B. Time frequency signal analysis and processing: a comprehensive reference. Boston: Elsevier; 2003.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

