Differential anti-tumour effects of MTH1 inhibitors in patient-derived 3D colorectal cancer cultures
- PMID: 30692572
- PMCID: PMC6349914
- DOI: 10.1038/s41598-018-37316-w
Differential anti-tumour effects of MTH1 inhibitors in patient-derived 3D colorectal cancer cultures
Abstract
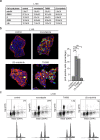
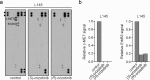
Reactive oxygen species (ROS) function as second messengers in signal transduction, but high ROS levels can also cause cell death. MTH1 dephosphorylates oxidized nucleotides, thereby preventing their incorporation into DNA and protecting tumour cells from oxidative DNA damage. Inhibitors of MTH1 (TH588 and (S)-crizotinib) were shown to reduce cancer cell viability. However, the MTH1-dependency of the anti-cancer effects of these drugs has recently been questioned. Here, we have assessed anti-tumour effects of TH588 and (S)-crizotinib in patient-derived 3D colorectal cancer cultures. Hypoxia and reoxygenation - conditions that increase intracellular ROS levels - increased sensitivity to (S)-crizotinib, but not to TH588. (S)-crizotinib reduced tyrosine phosphorylation of c-MET and ErbB3 whereas TH588 induced a mitotic cell cycle arrest, which was not affected by adding ROS-modulating compounds. Furthermore, we show that both compounds induced DNA damage that could not be prevented by adding the ROS inhibitor N-acetyl-L-cysteine. Moreover, adding ROS-modulating compounds did not alter the reduction in viability in response to TH588 and (S)-crizotinib. We conclude that TH588 and (S)-crizotinib have very clear and distinct anti-tumour effects in 3D colorectal cancer cultures, but that these effects most likely occur through distinct and ROS-independent mechanisms.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

