Herpes Simplex Virus Type 1 Enhances Expression of the Synaptic Protein Arc for Its Own Benefit
- PMID: 30692913
- PMCID: PMC6340317
- DOI: 10.3389/fncel.2018.00505
Herpes Simplex Virus Type 1 Enhances Expression of the Synaptic Protein Arc for Its Own Benefit
Abstract
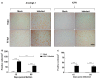
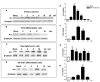
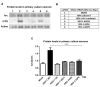
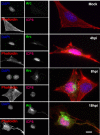
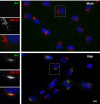
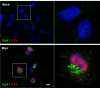
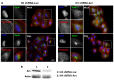
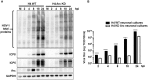
Herpes simplex virus type 1 (HSV-1) is a neurotropic virus able to reach the central nervous system (CNS) after primary infection in oronasal mucosa. HSV-1 establishes latency inside neurons due the repression of its gene expression process, which is related to periodic reactivations in response to cellular stress conditions, constituting a risk factor for neurodegenerative diseases such as Alzheimer's disease (AD). The immediate-early gene Arc plays an essential role in neuronal morphology, synaptic plasticity and memory formation. Arc acts as a hub protein, interacting with components of the endocytic machinery required for AMPA receptor (AMPAR) recycling as well as with proteins of the post-synaptic density and actin cytoskeleton. However, to date, no studies have evaluated whether persistent neurotropic HSV-1 infection modulates the expression or function of Arc protein in brain tissue. Here, we report that neuronal in vivo and in vitro infection of HSV-1 significantly increases Arc protein levels, showing a robust perinuclear distribution in neuronal cell lines, a process that is dependent on an active HSV-1 replication cycle. Finally, we found that silencing Arc protein caused a decrease in HSV-1 proteins and viral progeny, suggesting that Arc is involved in the lifecycle of HSV-1. Our studies strongly suggest that pathogenicity of HSV-1 neuronal reactivations in humans could be mediated in part by Arc neuronal upregulation and its potential role in endocytic trafficking and AMPA-neuronal function impairment. Further studies are necessary to define whether this phenomenon could have repercussions in cognition and learning processes in infected individuals.
Keywords: Alzheimer’s disease; Arc; HSV-1; neurodegeneration; neuronal dysfunction; neuronal infection; neurotropic virus.
Figures

References
LinkOut - more resources
Full Text Sources

