In silico Identification and Expression of Protocadherin Gene Family in Octopus vulgaris
- PMID: 30692932
- PMCID: PMC6339937
- DOI: 10.3389/fphys.2018.01905
In silico Identification and Expression of Protocadherin Gene Family in Octopus vulgaris
Abstract
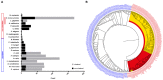
Connecting millions of neurons to create a functional neural circuit is a daunting challenge. Vertebrates developed a molecular system at the cell membrane to allow neurons to recognize each other by distinguishing self from non-self through homophilic protocadherin interactions. In mammals, the protocadherin gene family counts about 50 different genes. By hetero-multimerization, protocadherins are capable of generating an impressive number of molecular interfaces. Surprisingly, in the California two-spot octopus, Octopus bimaculoides, an invertebrate belonging to the Phylum Mollusca, over 160 protocadherins (PCDHs) have been identified. Here we briefly discuss the role of PCDHs in neural wiring and conduct a comparative study of the protocadherin gene family in two closely related octopus species, Octopus vulgaris and O. bimaculoides. A first glance at the expression patterns of protocadherins in O. vulgaris is also provided. Finally, we comment on PCDH evolution in the light of invertebrate nervous system plasticity.
Keywords: DSCAM; cephalopod; neural wiring; octopus; plasticity; protocadherins.
Figures

References
LinkOut - more resources
Full Text Sources

