Blockade of Store-Operated Calcium Entry Reduces IL-17/TNF Cytokine-Induced Inflammatory Response in Human Myoblasts
- PMID: 30693003
- PMCID: PMC6339936
- DOI: 10.3389/fimmu.2018.03170
Blockade of Store-Operated Calcium Entry Reduces IL-17/TNF Cytokine-Induced Inflammatory Response in Human Myoblasts
Abstract
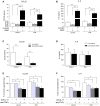
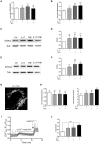
Muscle inflammation as in idiopathic inflammatory myopathies (IIM) leads to muscle weakness, mononuclear cell infiltration, and myofiber dysfunction affecting calcium channels. The effects of interleukin-17A (IL-17) and tumor necrosis factor-α (TNFα) on inflammation and calcium changes were investigated in human myoblasts. Human myoblasts were exposed to IL-17 and/or TNFα with/without store-operated Ca2+ entry (SOCE) inhibitors (2-ABP or BTP2). For co-cultures, peripheral blood mononuclear cells (PBMC) from healthy donors activated or not with phytohemagglutinin (PHA) were added to myoblasts at a 5:1 ratio. IL-17 and TNFα induced in synergy CCL20 and IL-6 production by myoblasts (>14-fold). PBMC-myoblast co-cultures enhanced CCL20 and IL-6 production in the presence or not of PHA compared to PBMC or myoblast monocultures. Anti-IL-17 and/or anti-TNFα decreased the production of IL-6 in co-cultures (p < 0.05). Transwell system that prevents direct cell-cell contact reduced CCL20 (p < 0.01) but not IL-6 secretion. IL-17 and/or TNFα increased the level of the ER stress marker Grp78, mitochondrial ROS and promoted SOCE activation by 2-fold (p < 0.01) in isolated myoblasts. SOCE inhibitors reduced the IL-6 production induced by IL-17/TNFα. Therefore, muscle inflammation induced by IL-17 and/or TNFα may increase muscle cell dysfunction, which, in turn, increased inflammation. Such close interplay between immune and non-immune mechanisms may drive and increase muscle inflammation and weakness.
Keywords: inflammatory myopathies; interleukin-17; myoblasts; store-operated calcium entry; tumor necrosis factor-α.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

