Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action
- PMID: 30693034
- PMCID: PMC6332982
- DOI: 10.1155/2018/2952085
Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action
Abstract
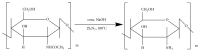
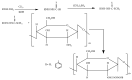
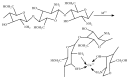
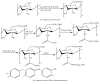
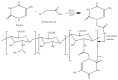
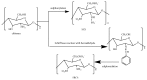
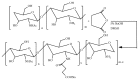
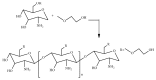
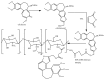
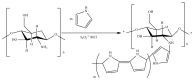
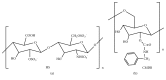
Tailoring of chitosan through the involvement of its amino, acetamido, and hydroxy groups can give derivatives of enhanced solubility and remarkable anticancer activity. The general mechanism of such activity is associated with the disturbances in normal functioning of cell cycle, interference to the central dogma of biological system from DNA to RNA to protein or enzymatic synthesis, and the disruption of hormonal path to biosynthesis to inhibit the growth of cancer cells. Both chitosan and its various derivatives have been reported to selectively permeate through the cancer cell membranes and show anticancer activity through the cellular enzymatic, antiangiogenic, immunoenhancing, antioxidant defense mechanism, and apoptotic pathways. They get sequestered from noncancer cells and provide their enhanced bioavailability in cancer cells in a sustained release manner. This review presents the putative mechanisms of anticancer activity of chitosan and mechanistic approaches of structure activity relation upon the modification of chitosan through functionalization, complex formation, and graft copolymerization to give different derivatives.
Figures



References
-
- Ramya R., Sudha P. N., Mahalakshmi J. Preparation and Characterization of Chitosan Binary Blend. International Journal of Scientific and Research Publications. 2012;2:1–9.
-
- Zhang Z. T., Chen D. H., Chen L. Preparation of two different serials of chitosan. Journal of Dong Hua University (English Edition) 2002;19:36–39.
-
- Gavhane Y. N., Gurav A. S., Yadav A. V. Chitosan and Its Applications: A Review of Literature. International journal of research in pharmaceutical and biomedical sciences. 2013;4(1):312–331.
-
- Ray S. D. Potential aspects of chitosan as pharmaceutical excipient. Acta Poloniae Pharmaceutica. 2011;68(5):619–622. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

