Rational Design and Construction of Cocatalysts for Semiconductor-Based Photo-Electrochemical Oxygen Evolution: A Comprehensive Review
- PMID: 30693190
- PMCID: PMC6343073
- DOI: 10.1002/advs.201801505
Rational Design and Construction of Cocatalysts for Semiconductor-Based Photo-Electrochemical Oxygen Evolution: A Comprehensive Review
Abstract
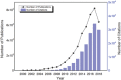
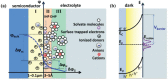
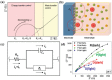
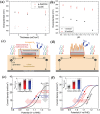
Photo-electrochemical (PEC) water splitting, as an essential and indispensable research branch of solar energy applications, has achieved increasing attention in the past decades. Between the two photoelectrodes, the photoanodes for PEC water oxidation are mostly studied for the facile selection of n-type semiconductors. Initially, the efficiency of the PEC process is rather limited, which mainly results from the existing drawbacks of photoanodes such as instability and serious charge-carrier recombination. To improve PEC performances, researchers gradually focus on exploring many strategies, among which engineering photoelectrodes with suitable cocatalysts is one of the most feasible and promising methods to lower reaction obstacles and boost PEC water splitting ability. Here, the basic principles, modules of the PEC system, evaluation parameters in PEC water oxidation reactions occurring on the surface of photoanodes, and the basic functions of cocatalysts on the promotion of PEC performance are demonstrated. Then, the key progress of cocatalyst design and construction applied to photoanodes for PEC oxygen evolution is emphatically introduced and the influences of different kinds of water oxidation cocatalysts are elucidated in detail. Finally, the outlook of highly active cocatalysts for the photosynthesis process is also included.
Keywords: cocatalysts; oxygen evolution reaction; photoanodes; solar energy; water splitting.
Figures


















References
-
- Zhang N., Han C., Fu X., Xu Y.‐J., Chem 2018, 4, 1832.
-
- Chen X., Li N., Kong Z., Ong W., Zhao X., Mater. Horiz. 2018, 5, 9.
-
- Alfaifi B. Y., Ullah H., Alfaifi S., Tahir A. A., Mallick T. K., Veruscript Funct. Nanomater. 2018, 2, BDJOC3.
-
- Rajeshwar K., J. Appl. Electrochem. 1995, 25, 1067.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
