Black and Brown Oro-facial Mucocutaneous Neoplasms
- PMID: 30693458
- PMCID: PMC6406009
- DOI: 10.1007/s12105-019-01008-2
Black and Brown Oro-facial Mucocutaneous Neoplasms
Abstract
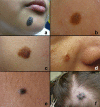
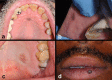
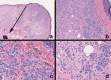
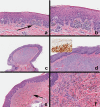
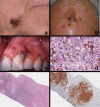
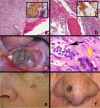
Black and brown-colored mucocutaneous lesions present a differential diagnostic challenge, with malignant melanoma being the primary clinical concern. The vast majority of pigmented lesions in the head and neck region are the result of benign, reactive factors such as post-inflammatory melanosis. However, it is not uncommon to discover a range of muco-cutaneous black and brown neoplasms in the oro-facial area. The majority of black/brown pigmented neoplasms are melanocytic in origin; these are neoplasms of neural crest derivation. Melanocytic nevi are a diverse group of benign neoplasms that are the result of specific oncogenic mutations. They are common on cutaneous surfaces but can manifest in mucosal sites. Currently, nevi are classified based on clinical and histological criteria. The most common cutaneous and oral mucosal nevus is the acquired melanocytic nevus; nevi do not pose an increased risk for the development of malignant melanoma. Emerging information on specific genetic differences supports the notion of biologically distinct nevi. This article will review the classic clinical and microscopic features of nevi commonly found in the head and neck region, and discuss emerging concepts in nevus pathogenesis and taxonomy. Melanoma is a malignant melanocytic neoplasm and is a result of cumulative genetic deregulation. The etiology of malignant melanoma (MM) is multifactorial and includes underlying genetic susceptibility, UV radiation, skin-type, and race. The majority of MM occurs on cutaneous surfaces and less commonly on mucosal and extra-cutaneous visceral organs. Regardless of location, MM exhibits clinical-pathological features that relate to horizontal or vertical tumor spread. Cutaneous and mucosal MM typically present as asymmetrical, irregularly bordered, large (> 0.5 cm), heterogeneous brown-black lesions with foci of erythema, atrophy or ulceration. As with melanocytic nevi, advances in melanomagenesis research have revealed primary oncogenic BRAF and NRAS mutations associated with cutaneous MM. Unlike their cutaneous counterparts, mucosal melanomas exhibit primary oncogenic alterations in c-KIT and other genes. This article will discuss the role of specific primary oncogenic and secondary/tertiary genetic defects in differential clinical presentation, anatomic distribution, future classification changes, and targeted therapy of melanoma. The clinical and microscopic features of mucosal melanomas and a summary of management guidelines will be discussed. Additionally, this article will cover the salient features of melanocytic neuroectodermal tumor of infancy, a neoplastic entity that can involve the oro-facial region, and the clinical-pathological features of selected, commonly occurring pigmented ectodermally-derived neoplasms that are often part of the clinical differential diagnosis of black-brown pigmented lesions.
Keywords: Melanocytic nevi; Melanoma; Oral melanocytic nevus; Oral melanoma; Pigmented neoplasm.
Conflict of interest statement
Conflict of interest
The author declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individuals whose photographs are used in this article.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

