Effect of pembrolizumab on CD4+ CD25+ , CD4+ LAP+ and CD4+ TIM-3+ T cell subsets
- PMID: 30693485
- PMCID: PMC6514373
- DOI: 10.1111/cei.13264
Effect of pembrolizumab on CD4+ CD25+ , CD4+ LAP+ and CD4+ TIM-3+ T cell subsets
Abstract
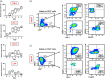
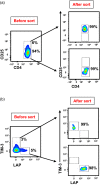
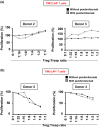
Tumor immune evasion involves the expansion of avidly proliferating immunosuppressive cells and inhibition of effector T cell proliferation. Immune checkpoints (IC) block the activation pathways of tumor-reactive T cells. IC pathways are often exploited by tumor cells to evade immune destruction, and blocking these pathways through IC inhibitors (ICI) has shown promising results in multiple malignancies. In this study, we investigated the effects of an ICI, pembrolizumab, on various T cell subsets in vitro. We compared the suppressive activity of CD4+ CD25+ regulatory T cells (conventional Treg ) with T cells expressing T cell immunoglobulin-3+ (TIM-3+ ) and latency-associated peptide (LAP)+ T cells. We found that LAP-expressing T cells were more suppressive than conventional Treg , but TIM-3-expressing T cells were not suppressive. Our results show that pembrolizumab does not modulate functions of Treg and mediates its immunostimulatory effects via the release of effector T cells from suppression. These findings may assist in the development of agents designed to intervene in IC pathways to overcome Treg resistance to ICI.
Keywords: cell activation; cell proliferation; regulatory T cells.
© 2019 British Society for Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

