B(C6F5)3-Catalyzed C-H Alkylation of N-Alkylamines Using Silicon Enolates without External Oxidant
- PMID: 30693779
- PMCID: PMC6591580
- DOI: 10.1021/acs.orglett.8b03959
B(C6F5)3-Catalyzed C-H Alkylation of N-Alkylamines Using Silicon Enolates without External Oxidant
Abstract
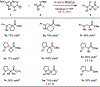
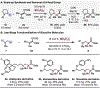
An efficient method for the coupling of N-alkylamines with silicon enolates to generate β-amino carbonyl compounds is disclosed. These reactions proceed by activation of α-amino C-H bonds by B(C6F5)3, which likely generates a "frustrated" acid/base complex in the presence of large N-alkylamines. The transformation requires no external oxidant and releases hydrosilane as a byproduct. The utility of this method is demonstrated in the late-stage functionalization of bioactive molecules such as citalopram, atomoxetine, and fluoxetine.
Figures



References
-
- For reviews on various types of Mannich-type reactions, see:
- Kobayashi S.; Ishitani H Catalytic Enantioselective Addition to Imines. Chem. Rev. 1999, 99, 1069–1094. - PubMed
- Córdova A The Direct Catalytic Asymmetric Mannich Reaction. Acc. Chem. Res. 2004, 37, 102–112. - PubMed
- Sodeoka M; Hamashima Y Development of Catalytic Enantioselective Reactions via Palladium Enolates as Key Intermediates. Bull. Chem. Soc. Jpn. 2005, 78, 941–956.
- Wenzel AG; Jacobsen EN in Enantioselective Synthesis of beta-Amino Acids, Juaristi E, Soloshonok V, Eds.; Wiley-VCH: New York, 2005; Chapter 4.
- Shibasaki M; Matsunaga S Metal/linked-BINOL complexes: Applications in direct catalytic asymmetric Mannich-type reactions. J. Organomet. Chem. 2006, 691, 2089–2100.
- Ting A; Schaus SE Organocatalytic Asymmetric Mannich Reactions: New Methodology, Catalyst Design, and Synthetic Applications. Eur. J. Org. Chem. 2007, 2007, 5797–5815.
- Verkade JMM; Hemert L. J. C.v..; Quaedflieg PJLM.; Rutjes FPJT Organocatalysed asymmetric Mannich Reaction. Chem. Soc. Rev. 2008, 37, 29–41. - PubMed
- Weiner B; Szymański W; Janssen DB; Minnaard AJ; Feringa BL Recent advances in the catalytic asymmetric synthesis of β-amino acids. Chem. Soc. Rev. 2010, 39, 1656–1691. - PubMed
- Karimi B; Enders D; Jafari E Recent Advances in Metal-Catalyzed Asymmetric Mannich Reactions. Synthesis 2013, 45, 2769–2812.
- Shirakawa S; Maruoka K Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem., Int. Ed. 2013, 52, 4312–4348. - PubMed
-
- For selected examples of oxidative Mannich-type reactions, see:
- Catino AJ; Nichols JM; Nettles BJ; Doyle MP The Oxidative Mannich Reaction Catalyzed by Dirhodium Caprolactamate. J. Am. Chem. Soc. 2006, 128, 5648–5649. - PMC - PubMed
- Sureshkumar D; Sud A; Klussmann M Aerobic Oxidative Coupling of Tertiary Amines with Silyl Enolates and Ketene Acetals. Synlett 2009, 2009, 1558–1561.
- Chu L; Zhang X; Qing F-L CuBr-Catalyzed Oxidative Difluoromethylation of Tertiary Amines with Difluoroenol Silyl Ethers. Org. Lett. 2009, 11, 2197–2200. - PubMed
- Sud A; Sureshkumar D; Klussmann M Oxidative coupling of amines and ketones by combined vanadium- and organocatalysis. Chem. Commun. 2009, 3169–3171. - PubMed
- Rueping M; Vila C; Koenigs RM; Poscharny K; Fabry DC Dual catalysis: combining photoredox and Lewis base catalysis for direct Mannich reactions. Chem. Commun. 2011, 47, 2360–2362. - PubMed
- Boess E; Sureshkumar D; Sud A; Wirtz C; Farès C; Klussmann M Mechanistic Studies on a Cu-Catalyzed Aerobic Oxidative Coupling Reaction with N-Phenyl Tetrahydroisoquinoline: Structure of Intermediates and the Role of Methanol As a Solvent. J. Am. Chem. Soc. 2011, 133, 8106–8109. - PubMed
- Boess E; Schmitz C; Klussmann M A Comparative Mechanistic Study of Cu-Catalyzed Oxidative Coupling Reactions with N-Phenyltetrahydroisoquinoline. J. Am. Chem. Soc. 2012, 134, 5317–5325. - PubMed
- Ratnikov MO; Doyle MP Mechanistic Investigation of Oxidative Mannich Reaction with tert-Butyl Hydroperoxide. The Role of Transition Metal Salt. J. Am. Chem. Soc. 2013, 135, 1549–1557. - PubMed
-
- For reviews of amine functionalization by catalytic oxidation of α-amino C–H bonds, see:
- Murahashi S; Zhang D. Ruthenium catalyzed biomimetic oxidation in organic synthesis inspired by cytochrome P-450. Chem. Soc. Rev. 2008, 37, 1490–1501. - PubMed
- Li C-J Cross-Dehydrogenative Coupling (CDC): Exploring C–C Bond Formations beyond Functional Group Transformations. Acc. Chem. Res. 2009, 42, 335–344. - PubMed
- Girard SA; Knauber T; Li C-J The Cross-Dehydrogenative Coupling of Csp3–H Bonds: A Versatile Strategy for C–C Bond Formations. Angew. Chem., Int. Ed. 2014, 53, 74–100. - PubMed
-
- For recent advances of oxidant-free Mannich-type reactions through α-amino C–H functionalization, see:
- Chen W.; Seidel D The Redox-Mannich Reaction. Org. Lett. 2014, 16, 3158–3161. - PMC - PubMed
- Ma L; Seidel D Intramolecular Redox-Mannich Reactions: Facile Access to the Tetrahydroprotoberberine Core. Chem. Eur. J. 2015, 21, 12908–12913. - PMC - PubMed
- Chen W; Seidel D Redox-Annulation of Cyclic Amines and β‐Ketoaldehydes. Org. Lett. 2016, 18, 1024–1027. - PMC - PubMed
-
- Chan JZ; Yao W; Hastings BT; Lok CK; Wasa M Direct Mannich-Type Reactions Promoted by Frustrated Lewis Acid/Bronsted Base Catalysts. Angew. Chem., Int. Ed. 2016, 55, 13877–13881. - PubMed
- Shang M; Cao M; Wang Q; Wasa M Enantioselective Direct Mannich-Type Reaction Catalyzed by Frustrated Lewis Acid/Brønsted Base Complexes. Angew. Chem., Int. Ed. 2017, 56, 13338–13341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

