Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial
- PMID: 30694322
- PMCID: PMC6439762
- DOI: 10.1001/jama.2018.21443
Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial
Abstract
Importance: Dexamethasone initiated after the first week of life reduces the rate of death or bronchopulmonary dysplasia (BPD) but may cause long-term adverse effects in very preterm infants. Hydrocortisone is increasingly used as an alternative, but evidence supporting its efficacy and safety is lacking.
Objective: To assess the effect of hydrocortisone initiated between 7 and 14 days after birth on death or BPD in very preterm infants.
Design, setting, and participants: Double-blind, placebo-controlled randomized trial conducted in 19 neonatal intensive care units in the Netherlands and Belgium from November 15, 2011, to December 23, 2016, among preterm infants with a gestational age of less than 30 weeks and/or birth weight of less than 1250 g who were ventilator dependent between 7 and 14 days of life, with follow-up to hospital discharge ending December 12, 2017.
Interventions: Infants were randomly assigned to receive a 22-day course of systemic hydrocortisone (cumulative dose, 72.5 mg/kg) (n = 182) or placebo (n = 190).
Main outcomes and measures: The primary outcome was a composite of death or BPD assessed at 36 weeks' postmenstrual age. Twenty-nine secondary outcomes were analyzed up to hospital discharge, including death and BPD at 36 weeks' postmenstrual age.
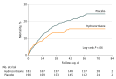
Results: Among 372 patients randomized (mean gestational age, 26 weeks; 55% male), 371 completed the trial; parents withdrew consent for 1 child treated with hydrocortisone. Death or BPD occurred in 128 of 181 infants (70.7%) randomized to hydrocortisone and in 140 of 190 infants (73.7%) randomized to placebo (adjusted risk difference, -3.6% [95% CI, -12.7% to 5.4%]; adjusted odds ratio, 0.87 [95% CI, 0.54-1.38]; P = .54). Of 29 secondary outcomes, 8 showed significant differences, including death at 36 weeks' postmenstrual age (15.5% with hydrocortisone vs 23.7% with placebo; risk difference, -8.2% [95% CI, -16.2% to -0.1%]; odds ratio, 0.59 [95% CI, 0.35-0.995]; P = .048). Twenty-one outcomes showed nonsignificant differences, including BPD (55.2% with hydrocortisone vs 50.0% with placebo; risk difference, 5.2% [95% CI, -4.9% to 15.2%]; odds ratio, 1.24 [95% CI, 0.82-1.86]; P = .31). Hyperglycemia requiring insulin therapy was the only adverse effect reported more often in the hydrocortisone group (18.2%) than in the placebo group (7.9%).
Conclusions and relevance: Among mechanically ventilated very preterm infants, administration of hydrocortisone between 7 and 14 days after birth, compared with placebo, did not improve the composite outcome of death or BPD at 36 weeks' postmenstrual age. These findings do not support the use of hydrocortisone for this indication.
Trial registration: Netherlands National Trial Register Identifier: NTR2768.
Conflict of interest statement
Figures

Comment in
-
Does hydrocortisone reduce death and BPD in preterm infants who remain intubated beyond a week of life?Acta Paediatr. 2020 Jul;109(7):1495-1496. doi: 10.1111/apa.15206. Epub 2020 Mar 13. Acta Paediatr. 2020. PMID: 32167184 No abstract available.
References
-
- Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi: 10.1001/jama.2015.10244 - DOI - PMC - PubMed
-
- De Jesus LC, Pappas A, Shankaran S, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Risk factors for post-neonatal intensive care unit discharge mortality among extremely low birth weight infants. J Pediatr. 2012;161(1):70-74. doi: 10.1016/j.jpeds.2011.12.038 - DOI - PMC - PubMed
-
- Malleske DT, Chorna O, Maitre NL. Pulmonary sequelae and functional limitations in children and adults with bronchopulmonary dysplasia. Paediatr Respir Rev. 2018;26:55-59. - PubMed
-
- Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361-367. doi: 10.1001/jamapediatrics.2017.5323 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

