Pharmacokinetic and Drug-Drug Interaction Profiles of the Combination of Tezacaftor/Ivacaftor
- PMID: 30694595
- PMCID: PMC6510372
- DOI: 10.1111/cts.12610
Pharmacokinetic and Drug-Drug Interaction Profiles of the Combination of Tezacaftor/Ivacaftor
Abstract
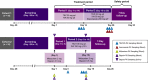
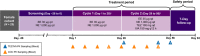
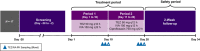
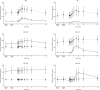
Drug-drug interaction (DDI) studies are described for tezacaftor/ivacaftor, a new cystic fibrosis transmembrane conductance regulator modulator therapy for the treatment of cystic fibrosis. Three phase I DDI studies were conducted in healthy subjects to characterize the DDI profile of tezacaftor/ivacaftor with cytochrome P450 (CYP)3A substrates, CYP3A inhibitors, and a permeability glycoprotein (P-gp) substrate. The effects of steady-state tezacaftor/ivacaftor on the pharmacokinetics (PKs) of digoxin (a P-gp substrate), midazolam, and ethinyl estradiol/norethindrone (CYP3A substrates) were evaluated. Effects of strong (itraconazole) and moderate (ciprofloxacin) CYP3A inhibitors on tezacaftor/ivacaftor PKs were also determined. Tezacaftor/ivacaftor increased digoxin area under the curve (AUC) by 30% but did not affect midazolam, ethinyl estradiol, or norethindrone exposures. Itraconazole increased the AUC of tezacaftor 4-fold and ivacaftor 15.6-fold. Ciprofloxacin had no significant effect on tezacaftor or ivacaftor exposure. Coadministration of tezacaftor/ivacaftor may increase exposure of sensitive P-gp substrates. Tezacaftor/ivacaftor is unlikely to impact exposure of drugs metabolized by CYP3A, including hormonal contraceptives. Strong CYP3A inhibitors significantly increase the exposures of tezacaftor and ivacaftor.
© 2019 Vertex Pharmaceuticals, Incorporated. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Varun Garg, Chonghua Li, Asfiha Gebre, Sarah Robertson, Licong Jiang, Kristin Stephan, Lakshmi Viswanathan, Jessica Parkinson, Linda T. Wang, and Julie Lekstrom‐Himes are employees of Vertex Pharmaceuticals, Inc. and may hold stock and/or stock options in the company. Jinshan Shen, Sagar Agarwal, Jiayin Huang, and Linda Han were employees of Vertex Pharmaceuticals, Inc. at the time of analysis and may hold stock and/or stock options in the company. As an Associate Editor for
Figures
References
-
- Cystic Fibrosis Foundation . Cystic Fibrosis Foundation Patient Registry 2015 Annual Data Report (Cystic Fibrosis Foundation, Bethesda, MD, 2016).
-
- Cystic Fibrosis Trust . UK Cystic Fibrosis Registry 2015 Annual Data Report (Cystic Fibrosis Trust, London, UK, 2016).
-
- Symdeko [package insert]. Boston, MA: Vertex Pharmaceuticals Incorporated; 2018.
-
- Taylor‐Cousar, J.L. et al Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N. Engl. J. Med. 377, 2013–2023 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

