Understanding the Effects of General Anesthetics on Cortical Network Activity Using Ex Vivo Preparations
- PMID: 30694851
- PMCID: PMC6520142
- DOI: 10.1097/ALN.0000000000002554
Understanding the Effects of General Anesthetics on Cortical Network Activity Using Ex Vivo Preparations
Abstract
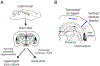
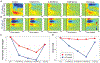
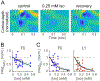
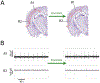
General anesthetics have been used to ablate consciousness during surgery for more than 150 yr. Despite significant advances in our understanding of their molecular-level pharmacologic effects, comparatively little is known about how anesthetics alter brain dynamics to cause unconsciousness. Consequently, while anesthesia practice is now routine and safe, there are many vagaries that remain unexplained. In this paper, the authors review the evidence that cortical network activity is particularly sensitive to general anesthetics, and suggest that disruption to communication in, and/or among, cortical brain regions is a common mechanism of anesthesia that ultimately produces loss of consciousness. The authors review data from acute brain slices and organotypic cultures showing that anesthetics with differing molecular mechanisms of action share in common the ability to impair neurophysiologic communication. While many questions remain, together, ex vivo and in vivo investigations suggest that a unified understanding of both clinical anesthesia and the neural basis of consciousness is attainable.
Figures


References
-
- Dingledine R, Dodd J, Kelly JS: The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J Neurosci Methods 1980; 2:323–62 - PubMed
-
- Cruikshank SJ, Rose HJ, Metherate R: Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol 2002; 87:361–84 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

