Antibody opsonization enhances MAIT cell responsiveness to bacteria via a TNF-dependent mechanism
- PMID: 30695101
- PMCID: PMC6767153
- DOI: 10.1111/imcb.12239
Antibody opsonization enhances MAIT cell responsiveness to bacteria via a TNF-dependent mechanism
Abstract
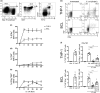
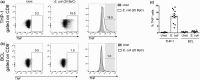
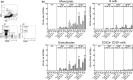
Mucosal-associated invariant T (MAIT) cells are an abundant human T-cell subset with antimicrobial properties. They can respond to bacteria presented via antigen-presenting cells (APCs) such as macrophages, which present bacterially derived ligands from the riboflavin synthesis pathway on MR1. Moreover, MAIT cells are also highly responsive to cytokines which enhance and even substitute for T-cell receptor-mediated signaling. The mechanisms leading to an efficient presentation of bacteria to MAIT cells by APCs have not been fully elucidated. Here, we showed that the monocytic cell line THP-1 and B cells activated MAIT cells differentially in response to Escherichia coli. THP-1 cells were generally more potent in inducing IFNγ and IFNγ/TNF production by MAIT cells. Furthermore, THP-1, but not B, cells produced TNF upon bacterial stimulation, which in turn supported IFNγ production by MAIT cells. Finally, we addressed the role of antibody-dependent opsonization of bacteria in the activation of MAIT cells using in vitro models. We found that opsonization had a substantial impact on downstream MAIT cell activation by monocytes. This was associated with enhanced activation of monocytes and increased TNF release. Importantly, this TNF acted in concert with other cytokines to drive MAIT cell activation. These data indicate both a significant interaction between adaptive and innate immunity in the response to bacteria, and an important role for TNF in MAIT cell triggering.
Keywords: TNF; Bacteria; E. coli; IgG-opsonization; MAIT cells; innate T cells.
© 2019 The Authors Immunology & Cell Biology published by John Wiley & Sons Australia, Ltd on behalf of Australasian Society for Immunology Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

