Corrosion of Metallic Biomaterials: A Review
- PMID: 30696087
- PMCID: PMC6384782
- DOI: 10.3390/ma12030407
Corrosion of Metallic Biomaterials: A Review
Abstract
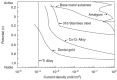
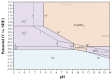
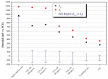
Metallic biomaterials are used in medical devices in humans more than any other family of materials. The corrosion resistance of an implant material affects its functionality and durability and is a prime factor governing biocompatibility. The fundamental paradigm of metallic biomaterials, except biodegradable metals, has been "the more corrosion resistant, the more biocompatible." The body environment is harsh and raises several challenges with respect to corrosion control. In this invited review paper, the body environment is analysed in detail and the possible effects of the corrosion of different biomaterials on biocompatibility are discussed. Then, the kinetics of corrosion, passivity, its breakdown and regeneration in vivo are conferred. Next, the mostly used metallic biomaterials and their corrosion performance are reviewed. These biomaterials include stainless steels, cobalt-chromium alloys, titanium and its alloys, Nitinol shape memory alloy, dental amalgams, gold, metallic glasses and biodegradable metals. Then, the principles of implant failure, retrieval and failure analysis are highlighted, followed by description of the most common corrosion processes in vivo. Finally, approaches to control the corrosion of metallic biomaterials are highlighted.
Keywords: biocompatibility; biodegradable metals; biomaterials; body environment; corrosion; failure; metallic glasses; shape memory alloys; stainless steels; titanium alloys.
Conflict of interest statement
The author declares no conflict of interest.
Figures




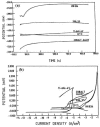
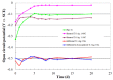
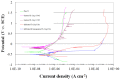







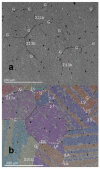

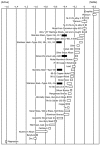


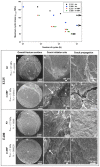


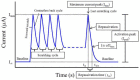
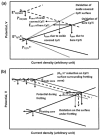



References
-
- Williams D.F., Black J., Doherty P.J. Consensus report of second conference on definitions in biomaterials. In: Doherty P.J., Williams R.L., Williams D.F., Lee A.J.C., editors. Biomaterial-Tissue Interfaces. Volume 10. Elsevier; Amsterdam, The Netherlands: 1992. pp. 525–533.
-
- Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E., editors. Biomaterials Science: An Introduction to Materials in Medicine. 2nd ed. Academic Press; San Diego, CA, USA: 2004.
-
- Williams D.F., editor. Definitions in Biomaterials—Proceedings of a Consensus Conference of the European Society Biomaterials. Volume 4 Elsevier; New York, NY, USA: 1987.
-
- Bronzino J.D., Peterson D.R., editors. The Biomedical Engineering Handbook. 4th ed. CRC Press; Boca Raton, FL, USA: 2015.
-
- Zierold A.A. Reaction of bone to various metals. Arch. Surg. 1924;9:365–412. doi: 10.1001/archsurg.1924.01120080133008. - DOI
Publication types
LinkOut - more resources
Full Text Sources

