Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression
- PMID: 30696103
- PMCID: PMC6387194
- DOI: 10.3390/ijms20030556
Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression
Abstract
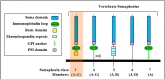
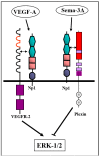
Abstract: Semaphorins are the products of a large gene family containing 28 genes of which 21 are found in vertebrates. Class-3 semaphorins constitute a subfamily of seven vertebrate semaphorins which differ from the other vertebrate semaphorins in that they are the only secreted semaphorins and are distinguished from other semaphorins by the presence of a basic domain at their C termini. Class-3 semaphorins were initially characterized as axon guidance factors, but have subsequently been found to regulate immune responses, angiogenesis, lymphangiogenesis, and a variety of additional physiological and developmental functions. Most class-3 semaphorins transduce their signals by binding to receptors belonging to the neuropilin family which subsequently associate with receptors of the plexin family to form functional class-3 semaphorin receptors. Recent evidence suggests that class-3 semaphorins also fulfill important regulatory roles in multiple forms of cancer. Several class-3 semaphorins function as endogenous inhibitors of tumor angiogenesis. Others were found to inhibit tumor metastasis by inhibition of tumor lymphangiogenesis, by direct effects on the behavior of tumor cells, or by modulation of immune responses. Notably, some semaphorins such as sema3C and sema3E have also been found to potentiate tumor progression using various mechanisms. This review focuses on the roles of the different class-3 semaphorins in tumor progression.
Keywords: angiogenesis; cancer; lymphangiogenesis; neuropilins; plexins; semaphorins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The semaphorins and their receptors as modulators of tumor progression.Drug Resist Updat. 2016 Nov;29:1-12. doi: 10.1016/j.drup.2016.08.001. Epub 2016 Aug 28. Drug Resist Updat. 2016. PMID: 27912840 Review.
-
The role of the semaphorins in cancer.Cell Adh Migr. 2016 Nov;10(6):652-674. doi: 10.1080/19336918.2016.1197478. Epub 2016 Aug 17. Cell Adh Migr. 2016. PMID: 27533782 Free PMC article. Review.
-
Semaphorins in cancer.Front Biosci. 2005 Jan 1;10:751-60. doi: 10.2741/1569. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15569615 Review.
-
Complexes of plexin-A4 and plexin-D1 convey semaphorin-3C signals to induce cytoskeletal collapse in the absence of neuropilins.J Cell Sci. 2018 May 4;131(9):jcs208298. doi: 10.1242/jcs.208298. J Cell Sci. 2018. PMID: 29661844
-
Semaphorin signaling in vascular and tumor biology.Adv Exp Med Biol. 2007;600:118-31. doi: 10.1007/978-0-387-70956-7_10. Adv Exp Med Biol. 2007. PMID: 17607951 Review.
Cited by
-
Neuropilin (NRPs) Related Pathological Conditions and Their Modulators.Int J Mol Sci. 2022 Jul 29;23(15):8402. doi: 10.3390/ijms23158402. Int J Mol Sci. 2022. PMID: 35955539 Free PMC article. Review.
-
Differences in the Expression Pattern of mRNA Protein SEMA3F in Endometrial Cancer in vitro under Cisplatin Treatment.Curr Pharm Biotechnol. 2020;21(11):1119-1128. doi: 10.2174/1389201021666200416102540. Curr Pharm Biotechnol. 2020. PMID: 32297576 Free PMC article.
-
Glomerular Endothelial Cells Are the Coordinator in the Development of Diabetic Nephropathy.Front Med (Lausanne). 2021 Jun 18;8:655639. doi: 10.3389/fmed.2021.655639. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34222276 Free PMC article.
-
Tissue-Targeted Transcriptomics Reveals SEMA3D Control of Hypoglossal Nerve Projection to Mouse Tongue Primordia.Acta Histochem Cytochem. 2024 Feb 29;57(1):35-46. doi: 10.1267/ahc.23-00073. Epub 2024 Feb 23. Acta Histochem Cytochem. 2024. PMID: 38463205 Free PMC article.
-
Characterization of Class-3 Semaphorin Receptors, Neuropilins and Plexins, as Therapeutic Targets in a Pan-Cancer Study.Cancers (Basel). 2020 Jul 6;12(7):1816. doi: 10.3390/cancers12071816. Cancers (Basel). 2020. PMID: 32640719 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

