Cerebellum, Predictions and Errors
- PMID: 30697149
- PMCID: PMC6340992
- DOI: 10.3389/fncel.2018.00524
Cerebellum, Predictions and Errors
Abstract
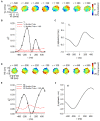
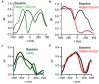
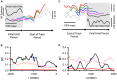
Making predictions and validating the predictions against actual sensory information is thought to be one of the most fundamental functions of the nervous system. A growing body of evidence shows that the neural mechanisms controlling behavior, both in motor and non-motor domains, rely on prediction errors, the discrepancy between predicted and actual information. The cerebellum has been viewed as a key component of the motor system providing predictions about upcoming movements and receiving feedback about motor errors. Consequentially, studies of cerebellar function have focused on the motor domain with less consideration for the wider context in which movements are generated. However, motor learning experiments show that cognition makes important contributions to motor adaptation that involves the cerebellum. One of the more successful theoretical frameworks for understanding motor control and cerebellar function is the forward internal model which states that the cerebellum predicts the sensory consequences of the motor commands and is involved in computing sensory prediction errors by comparing the predictions to the sensory feedback. The forward internal model was applied and tested mainly for effector movements, raising the question whether cerebellar encoding of behavior reflects task performance measures associated with cognitive involvement. Electrophysiological studies based on pseudo-random tracking in monkeys show that the discharge of Purkinje cell, the sole output neurons of the cerebellar cortex, encodes predictive and feedback signals not only of the effector kinematics but also of task performance. The implications are that the cerebellum implements both effector and task performance forward models and the latter are consistent with the cognitive contributions observed during motor learning. The implications of these findings include insights into recent psychophysical observations on moving with reduced feedback and motor learning. The findings also support the cerebellum's place in hierarchical generative models that work in concert to refine predictions about behavior and the world. Therefore, cerebellar representations bridge motor and non-motor domains and provide a better understanding of cerebellar function within the functional architecture of the brain.
Keywords: Purkinje cell; complex spike; forward internal model; generative model; kinematics; performance error; sensory prediction error; simple spike.
Figures

References
-
- Albus J. S. (1971). A theory of cerebellar function. Math. Biosci. 10, 25–61. 10.1016/0025-5564(71)90051-4 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

