Promoting Shewanella Bidirectional Extracellular Electron Transfer for Bioelectrocatalysis by Electropolymerized Riboflavin Interface on Carbon Electrode
- PMID: 30697199
- PMCID: PMC6340934
- DOI: 10.3389/fmicb.2018.03293
Promoting Shewanella Bidirectional Extracellular Electron Transfer for Bioelectrocatalysis by Electropolymerized Riboflavin Interface on Carbon Electrode
Abstract
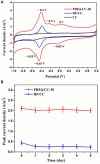
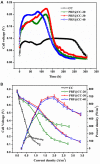
The extracellular electron transfer (EET) that connects the intracellular metabolism of electroactive microorganisms to external electron donors/acceptors, is the foundation to develop diverse microbial electrochemical technologies. For a particular microbial electrochemical device, the surface chemical property of an employed electrode material plays a crucial role in the EET process owing to the direct and intimate biotic-abiotic interaction. The functional modification of an electrode surface with redox mediators has been proposed as an effectual approach to promote EET, but the underlying mechanism remains unclear. In this work, we investigated the enhancement of electrochemically polymerized riboflavin interface on the bidirectional EET of Shewanella putrefaciens CN32 for boosting bioelectrocatalytic ability. An optimal polyriboflavin functionalized carbon cloth electrode achieved about 4.3-fold output power density (∼707 mW/m2) in microbial fuel cells and 3.7-fold cathodic current density (∼0.78 A/m2) for fumarate reduction in three-electrode cells compared to the control, showing great increases in both outward and inward EET rates. Likewise, the improvement was observed for polyriboflavin-functionalized graphene electrodes. Through comparison between wild-type strain and outer-membrane cytochrome (MtrC/UndA) mutant, the significant improvements were suggested to be attributed to the fast interfacial electron exchange between the polyriboflavin interface with flexible electrochemical activity and good biocompatibility and the outer-membrane cytochromes of the Shewanella strain. This work not only provides an effective approach to boost microbial electrocatalysis for energy conversion, but also offers a new demonstration of broadening the applications of riboflavin-functionalized interface since the widespread contribution of riboflavin in various microbial EET pathways together with the facile electropolymerization approach.
Keywords: Shewanella; bioelectrocatalysis; electropolymerization; extracellular electron transfer; riboflavin.
Figures


References
-
- Carmona-Martinez A. A., Harnisch F., Fitzgerald L. A., Biffinger J. C., Ringeisen B. R., Schröder U. (2011). Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants. Bioelectrochemistry 81 74–80. 10.1016/j.bioelechem.2011.02.006 - DOI - PubMed
-
- Celiesiute R., Radzevic A., Zukauskas A., Vaitekonis S., Pauliukaite R. (2017). A strategy to employ polymerised riboflavin in the development of electrochemical biosensors. Electroanalysis 29 2071–2082. 10.1002/elan.201700218 - DOI
-
- Chatterjee A., Foord J. S. (2009). Biological applications of diamond electrodes; electrochemical studies of riboflavin. Diam. Relat. Mater. 18 899–903. 10.1016/j.diamond.2009.02.016 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

