Dietary Habits and Intestinal Immunity: From Food Intake to CD4+ T H Cells
- PMID: 30697217
- PMCID: PMC6340974
- DOI: 10.3389/fimmu.2018.03177
Dietary Habits and Intestinal Immunity: From Food Intake to CD4+ T H Cells
Abstract
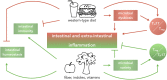
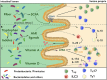
Dietary habits have a profound impact on intestinal homeostasis and in general on human health. In Western countries, high intake of calories derived from fried products, butter and processed meat is favored over dietary regimens rich in fruits and vegetables. This type of diet is usually referred to as Western-type diet (WTD) and it has been associated with several metabolic and chronic inflammatory conditions of the gastrointestinal tract. In this review, we describe how WTD promotes intestinal and extra-intestinal inflammation and alters mucosal immunity acting on CD4+ T cells in a microbiota-dependent or -independent fashion, ultimately leading to higher susceptibility to infectious and autoimmune diseases. Moreover, summarizing recent findings, we propose how dietary supplementation with fiber and vitamins could be used as a tool to modulate CD4+ T cell phenotype and function, ameliorating inflammation and restoring mucosal homeostasis.
Keywords: CD4 T cells; fat; fiber; inflammation; microbiota; mucosal immunity; salt; western diet.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

