The Role of Dietary Nutrients in Inflammatory Bowel Disease
- PMID: 30697218
- PMCID: PMC6340967
- DOI: 10.3389/fimmu.2018.03183
The Role of Dietary Nutrients in Inflammatory Bowel Disease
Abstract
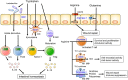
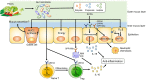
Inflammatory bowel disease (IBD) is a chronic and relapsing inflammatory disease of the gastrointestinal tract. Although the precise etiology of IBD remains incompletely understood, accumulating evidence suggests that various environmental factors, including dietary nutrients, contribute to its pathogenesis. Dietary nutrients are known to have an impact on host physiology and diseases. The interactions between dietary nutrients and intestinal immunity are complex. Dietary nutrients directly regulate the immuno-modulatory function of gut-resident immune cells. Likewise, dietary nutrients shape the composition of the gut microbiota. Therefore, a well-balanced diet is crucial for good health. In contrast, the relationships among dietary nutrients, host immunity and/or the gut microbiota may be perturbed in the context of IBD. Genetic predispositions and gut dysbiosis may affect the utilization of dietary nutrients. Moreover, the metabolism of nutrients in host cells and the gut microbiota may be altered by intestinal inflammation, thereby increasing or decreasing the demand for certain nutrients necessary for the maintenance of immune and microbial homeostasis. Herein, we review the current knowledge of the role dietary nutrients play in the development and the treatment of IBD, focusing on the interplay among dietary nutrients, the gut microbiota and host immune cells. We also discuss alterations in the nutritional metabolism of the gut microbiota and host cells in IBD that can influence the outcome of nutritional intervention. A better understanding of the diet-host-microbiota interactions may lead to new therapeutic approaches for the treatment of IBD.
Keywords: diet; immunity; inflammatory bowel disease (IBD); intestinal barrier; microbiota.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

