A Series of Analogues to the AT2R Prototype Antagonist C38 Allow Fine Tuning of the Previously Reported Antagonist Binding Mode
- PMID: 30697513
- PMCID: PMC6346239
- DOI: 10.1002/open.201800282
A Series of Analogues to the AT2R Prototype Antagonist C38 Allow Fine Tuning of the Previously Reported Antagonist Binding Mode
Abstract
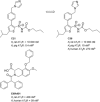
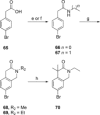
We here report on our continued studies of ligands binding to the promising drug target angiotensin II type 2 receptor (AT2R). Two series of compounds were synthesized and investigated. The first series explored the effects of adding small substituents to the phenyl ring of the known selective nonpeptide AT2R antagonist C38, generating small but significant shifts in AT2R affinity. One compound in the first series was equipotent to C38 and showed similar kinetic solubility, and stability in both human and mouse liver microsomes. The second series was comprised of new bicyclic derivatives, amongst which one ligand exhibited a five-fold improved affinity to AT2R as compared to C38. The majority of the compounds in the second series, including the most potent ligand, were inferior to C38 with regard to stability in both human and mouse microsomes. In contrast to our previously reported findings, ligands with shorter carbamate alkyl chains only demonstrated slightly improved stability in microsomes. Based on data presented herein, a more adequate, tentative model of the binding modes of ligand analogues to the prototype AT2R antagonist C38 is proposed, as deduced from docking redefined by molecular dynamic simulations.
Keywords: AT2 receptor; angiotensin II; medicinal chemistry; molecular docking; prototype antagonist.
Figures





References
-
- Hallberg M., Med. Res. Rev. 2015, 35, 464–519. - PubMed
-
- Whitebread S., Mele M., Kamber B., de Gasparo M., Biochem. Biophys. Res. Commun. 1989, 163, 284–291. - PubMed
-
- Chiu A. T., Herblin W. F., McCall D. E., Ardecky R. J., Carini D. J., Duncia J. V., Pease L. J., Wong P. C., Wexler R. R., Johnson A. L., Biochem. Biophys. Res. Commun. 1989, 165, 196–203. - PubMed
-
- Foulquier S., Steckelings U. M., Unger T., Nature 2013, 493, S9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

