Venlafaxine stimulates PNN proteolysis and MMP-9-dependent enhancement of gamma power; relevance to antidepressant efficacy
- PMID: 30697747
- PMCID: PMC6516074
- DOI: 10.1111/jnc.14671
Venlafaxine stimulates PNN proteolysis and MMP-9-dependent enhancement of gamma power; relevance to antidepressant efficacy
Abstract
Drugs that target monoaminergic transmission represent a first-line treatment for major depression. Though a full understanding of the mechanisms that underlie antidepressant efficacy is lacking, evidence supports a role for enhanced excitatory transmission. This can occur through two non-mutually exclusive mechanisms. The first involves increased function of excitatory neurons through relatively direct mechanisms such as enhanced dendritic arborization. Another mechanism involves reduced inhibitory function, which occurs with the rapid antidepressant ketamine. Consistent with this, GABAergic interneuron-mediated cortical inhibition is linked to reduced gamma oscillatory power, a rhythm also diminished in depression. Remission of depressive symptoms correlates with restoration of gamma power. As a result of strong excitatory input, reliable GABA release, and fast firing, PV-expressing neurons (PV neurons) represent critical pacemakers for synchronous oscillations. PV neurons also represent the predominant GABAergic population enveloped by perineuronal nets (PNNs), lattice-like structures that localize glutamatergic input. Disruption of PNNs reduces PV excitability and enhances gamma activity. Studies suggest that monoamine reuptake inhibitors reduce integrity of the PNN. Mechanisms by which these inhibitors reduce PNN integrity, however, remain largely unexplored. A better understanding of these issues might encourage development of therapeutics that best up-regulate PNN-modulating proteases. We observe that the serotonin/norepinephrine reuptake inhibitor venlafaxine increases hippocampal matrix metalloproteinase (MMP)-9 levels as determined by ELISA and concomitantly reduces PNN integrity in murine hippocampus as determined by analysis of sections following their staining with a fluorescent PNN-binding lectin. Moreover, venlafaxine-treated mice (30 mg/kg/day) show an increase in carbachol-induced gamma power in ex vivo hippocampal slices as determined by local field potential recording and Matlab analyses. Studies with mice deficient in matrix metalloproteinase 9 (MMP-9), a protease linked to PNN disruption in other settings, suggest that MMP-9 contributes to venlafaxine-enhanced gamma power. In conclusion, our results support the possibility that MMP-9 activity contributes to antidepressant efficacy through effects on the PNN that may in turn enhance neuronal population dynamics involved in mood and/or memory. Cover Image for this issue: doi: 10.1111/jnc.14498.
Keywords: MMP-9; depression; gamma oscillations; interneuron; perineuronal net; venlafaxine.
© 2019 International Society for Neurochemistry.
Figures

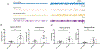

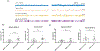
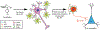
References
-
- Andersen P, Morris R, Amaral D, Bliss T, and O’Keefe J eds. (2006) The Hippocampus Book New York: Oxford University Press, 2006; Oxford Scholarship Online, 2009. doi: 10.1093/acprof:oso/9780195100273.001.0001. - DOI
-
- Arikan MK, Metin B and Tarhan N (2018) EEG gamma synchronization is associated with response to paroxetine treatment. J Affect Disord, 235, 114–116. - PubMed
-
- Bijata M, Labus J, Guseva D et al. (2017) Synaptic Remodeling Depends on Signaling between Serotonin Receptors and the Extracellular Matrix. Cell Rep, 19, 1767–1782. - PubMed
-
- Carulli D, Pizzorusso T, Kwok JC et al. (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain, 133, 2331–2347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

