Keratin 23 Is a Peroxisome Proliferator-Activated Receptor Alpha-Dependent, MYC-Amplified Oncogene That Promotes Hepatocyte Proliferation
- PMID: 30697791
- PMCID: PMC6597269
- DOI: 10.1002/hep.30530
Keratin 23 Is a Peroxisome Proliferator-Activated Receptor Alpha-Dependent, MYC-Amplified Oncogene That Promotes Hepatocyte Proliferation
Abstract
Chronic activation of the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARA) promotes MYC-linked hepatocellular carcinoma (HCC) in mice. Recent studies have shown that MYC can function as an amplifier of transcription where MYC does not act as an "on-off" switch for gene expression but rather accelerates transcription rates at active promoters by stimulating transcript elongation. Considering the possibility that MYC may amplify the expression of PPARA target genes to potentiate cell proliferation and liver cancer, gene expression was analyzed from livers of wild-type and liver-specific Myc knockout (MycΔHep ) mice treated with the PPARA agonist pirinixic acid. A subset of PPARA target genes was amplified in the presence of MYC, including keratin 23 (Krt23). The induction of Krt23 was significantly attenuated in MycΔHep mice and completely abolished in Ppara-null mice. Reporter gene assays and chromatin immunoprecipitation confirmed direct binding of both PPARA and MYC to sites within the Krt23 promoter. Forced expression of KRT23 in primary hepatocytes induced cell cycle-related genes. These data indicate that PPARA activation elevates MYC expression, which in turn potentiates the expression of select PPARA target genes involved in cell proliferation. Finally, KRT23 protein is highly elevated in human HCCs. Conclusion: These results revealed that MYC-mediated transcriptional potentiation of select PPARA target genes, such as Krt23, may remove rate-limiting constraints on hepatocyte growth and proliferation leading to liver cancer.
© 2019 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures



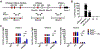

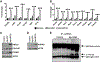


References
Publication types
MeSH terms
Substances
Grants and funding
- CIT&TCD20150325/The Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions/International
- Z01 BC005561/ImNIH/Intramural NIH HHS/United States
- CA/NCI NIH HHS/United States
- 81370521, 81670400 and 91739120/National Natural Science Foundation of China/International
- HI17C2082/Ministry of Health & Welfare, Republic of Korea/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

