Efficacy and safety of pneumatic dilation in achalasia: A systematic review and meta-analysis
- PMID: 30697952
- PMCID: PMC6849773
- DOI: 10.1111/nmo.13548
Efficacy and safety of pneumatic dilation in achalasia: A systematic review and meta-analysis
Abstract
Background and aims: One of the most used treatments for achalasia is pneumatic dilation of the lower esophageal sphincter to improve esophageal emptying. Multiple treatment protocols have been described with a varying balloon size, number of dilations, inflation pressure, and duration. We aimed to identify the most efficient and safe treatment protocol.
Methods: We performed a systematic review and meta-analysis of studies on pneumatic dilation in patients with primary achalasia. Clinical remission was defined as an Eckardt score ≤3 or adequate symptom reduction measured with a similar validated questionnaire. We compared the clinical remission rates and occurrence of complications between different treatment protocols.
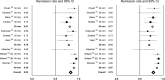
Results: We included 10 studies with 643 patients. After 6 months, dilation with a 30-mm or 35-mm balloon gave comparable mean success rates (81% and 79%, respectively), whereas a series of dilations up to 40 mm had a higher success rate of 90%. Elective additional dilation in patients with insufficient symptom resolution was somewhat more effective than performing a predefined series of dilations: 86% versus 75% after 12 months. Perforations occurred most often during initial dilations, and significantly more often using a 35-mm balloon than a 30-mm balloon (3.2 vs 1.0%); P = 0.027. A subsequent 35-mm dilation was safer than an initial dilation with 35 mm (0.97% vs 9.3% perforations), P = 0.0017.
Conclusions: The most efficient and safe method of dilating achalasia patients is a graded approach starting with a 30-mm dilation, followed by an elective 35-mm dilation and 40 mm when there is insufficient symptom relief.
Keywords: achalasia; balloon dilation; efficacy; safety.
© 2019 The Authors. Neurogastroenterology & Motility Published by John Wiley & Sons Ltd.
Conflict of interest statement
AB received research funding from Endostim, Medical Measurement Systems, Danone and given and received speaker and/or consulting fees from MMS, Astellas, AstraZeneca and Almirall. LP, FH, and AS have no conflict of interest to declare.
Figures


References
-
- Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA. 2015;313(18):1841‐1852. - PubMed
-
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108(8):1238‐1249. - PubMed
-
- Hirano I. Pathophysiology of achalasia. Curr Gastroenterol Rep. 1999;1(3):198‐202. - PubMed
-
- Mikaeli J, Bishehsari F, Montazeri G, Yaghoobi M, Malekzadeh R. Pneumatic balloon dilatation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther. 2004;20(4):431‐436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

