The optimal incubation time for in vitro hemocompatibility testing: Assessment using polymer reference materials under pulsatile flow with physiological wall shear stress conditions
- PMID: 30697956
- PMCID: PMC6767118
- DOI: 10.1002/jbm.b.34326
The optimal incubation time for in vitro hemocompatibility testing: Assessment using polymer reference materials under pulsatile flow with physiological wall shear stress conditions
Abstract
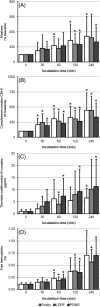
During hemocompatibility testing, activation products may reach plateau values which can result in less distinction between hemocompatible and hemo-incompatible materials. Of concern is an underestimation of the blood activation caused by the biomaterial of interest, which may result in a false assessment of hemocompatibility. To elucidate the optimal incubation time for in vitro hemocompatibility testing, we used the Haemobile circulation model with human whole blood. Blood from healthy volunteers was in vitro incubated under pulsatile flow with physiological wall shear stress conditions at 37°C for 30, 60, 120, or 240 min. Test loops containing low-density polyethylene and polydimethylsiloxane served as low and high reference materials, that is, hemocompatible and hemo-incompatible biomaterials, respectively. In addition, empty loops served as a negative reference. Thrombogenicity, platelet function, inflammatory response, coagulation, and hemolysis between references and incubation times were compared. We found that thrombogenicity and platelet function were significantly affected by both the duration of incubation and the type of material. In particular, thrombogenicity and platelet function assessments were affected by incubation time. We found that an exposure time of 60 min was sufficient, and for almost all variables an optimal incubation time to discriminate between the low and high reference material. © 2019 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater 107B: 2335-2342, 2019.
Keywords: biomaterials; blood-material interaction; cardiovascular device; hemocompatibility; hemocompatibility testing.
© 2019 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals, Inc.
Figures





References
-
- Blok SLJ, Engels GE, van Oeveren W. In vitro hemocompatibility testing: The importance of fresh blood. Biointerphases 2016;11:029802. - PubMed
-
- International Organization for Standardization . ISO 10993, Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. Genève: ISO; 2017.
-
- Seyfert UT, Biehl V, Schenk J. In vitro hemocompatibility testing of biomaterials according to the ISO 10993‐4. Biomol Eng 2002;19:91–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

