Effect of antidepressant switching between nortriptyline and escitalopram after a failed first antidepressant treatment among patients with major depressive disorder
- PMID: 30698114
- PMCID: PMC6624130
- DOI: 10.1192/bjp.2018.302
Effect of antidepressant switching between nortriptyline and escitalopram after a failed first antidepressant treatment among patients with major depressive disorder
Abstract
Background: For patients with major depressive disorder (MDD) experiencing side-effects or non-response to their first antidepressant, little is known regarding the effect of switching between a tricyclic antidepressant (TCA) and a selective serotonin reuptake inhibitor (SSRI).AimsTo compare the switch between the TCA nortriptyline and the SSRI escitalopram.
Method: Among 811 adults with MDD treated with nortriptyline or escitalopram for up to 12 weeks, 108 individuals switched from nortriptyline to escitalopram or vice versa because of side-effects or non-response (trial registration: EudraCT No.2004-001723-38 (https://eudract.ema.europa.eu/) and ISRCTN No.03693000 (http://www.controlled-trials.com)). Patients were followed for up to 26 weeks after switching and response was measured with the Montgomery-Åsberg Depression Rating scale (MADRS). We performed adjusted mixed-effects linear regression models with full information maximum likelihood estimation reporting β-coefficients with 95% CIs.
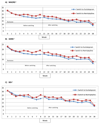
Results: Switching antidepressants resulted in a significant decrease in MADRS scores. This was present for switchers from escitalopram to nortriptyline (n = 36, β = -0.38, 95% CI -0.51 to -0.25, P<0.001) and from nortriptyline to escitalopram (n = 72, β = -0.34, 95% CI -0.41 to -0.26, P<0.001). Both switching options resulted in significant improvement among individuals who switched because of non-response or side-effects. The results were supported by analyses on other rating scales and symptom dimensions.
Conclusions: These results suggest that switching from a TCA to an SSRI or vice versa after non-response or side-effects to the first antidepressant may be a viable approach to achieve response among patients with MDD.Declarations of interestK.J.A. holds an Alberta Centennial Addiction and Mental Health Research Chair, funded by the Government of Alberta. K.J.A. has been a member of various advisory boards, received consultancy fees and honoraria, and has received research grants from various companies including Johnson and Johnson Pharmaceuticals Research and Development and Bristol-Myers Squibb Pharmaceuticals Limited. D.S. has served on advisory boards for, and received unrestricted grants from, Lundbeck and AstraZeneca. A.F. and P.M. have received honoraria for participating in expert panels for Lundbeck and GlaxoSmithKline.
Keywords: Depression; antidepressants; non-responders; side-effects; switching.
Conflict of interest statement
Dr. Aitchison holds an Alberta Centennial Addiction and Mental Health Research Chair, funded by the Government of Alberta. Dr. Aitchison has been a member of various advisory boards, received consultancy fees and honoraria, and has received research grants from various companies including Johnson and Johnson Pharmaceuticals Research and Development and Bristol-Myers Squibb Pharmaceuticals Limited. Dr. Souery has served on advisory boards for, and received unrestricted grants from, Lundbeck and AstraZeneca. Drs. Farmer and McGuffin have received honoraria for participating in expert panels for Lundbeck and GlaxoSmithKline. The other authors report no financial relationships with commercial interests.
Figures

Comment in
-
Unjustified conclusions.Br J Psychiatry. 2020 Jun;216(6):345. doi: 10.1192/bjp.2020.23. Br J Psychiatry. 2020. PMID: 32452340 No abstract available.
-
Authors' reply.Br J Psychiatry. 2020 Jun;216(6):345-346. doi: 10.1192/bjp.2020.24. Br J Psychiatry. 2020. PMID: 32452341 No abstract available.
References
-
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 163/1/28 [pii] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

