Biosynthesis and Chemical Applications of Thioamides
- PMID: 30698414
- PMCID: PMC6404778
- DOI: 10.1021/acschembio.8b01022
Biosynthesis and Chemical Applications of Thioamides
Abstract
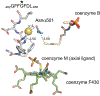
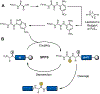
Thioamidation as a posttranslational modification is exceptionally rare, with only a few reported natural products and exactly one known protein example (methyl-coenzyme M reductase from methane-metabolizing archaea). Recently, there has been significant progress in elucidating the biosynthesis and function of several thioamide-containing natural compounds. Separate developments in the chemical installation of thioamides into peptides and proteins have enabled cell biology and biophysical studies to advance the current understanding of natural thioamides. This review highlights the various strategies used by Nature to install thioamides in peptidic scaffolds and the potential functions of this rare but important modification. We also discuss synthetic methods used for the site-selective incorporation of thioamides into polypeptides with a brief discussion of the physicochemical implications. This account will serve as a foundation for the further study of thioamides in natural products and their various applications.
Figures












References
-
- Patani GA; LaVoie EJ Bioisosterism: A Rational Approach in Drug Design. Chem. Rev 1996, 96 (8), 3147–3176. - PubMed
-
- Lee H-J; Choi Y-S; Lee K-B; Park J; Yoon C-J Hydrogen Bonding Abilities of Thioamide. J. Phys. Chem. A 2002, 106 (30), 7010–7017.
-
- Reiner A; Wildemann D; Fischer G; Kiefhaber T Effect of Thioxopeptide Bonds on α-Helix Structure and Stability. J. Am. Chem. Soc 2008, 130 (25), 8079–8084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

