Development of a Terpene Feedstock-Based Oxidative Synthetic Approach to the Illicium Sesquiterpenes
- PMID: 30698435
- PMCID: PMC6563921
- DOI: 10.1021/jacs.8b12247
Development of a Terpene Feedstock-Based Oxidative Synthetic Approach to the Illicium Sesquiterpenes
Abstract
The Illicium sesquiterpenes are a family of natural products containing over 100 highly oxidized and structurally complex members, many of which display interesting biological activities. This comprehensive account chronicles the evolution of a semisynthetic strategy toward these molecules from (+)-cedrol, seeking to emulate key aspects of their presumed biosynthesis. An initial route generated lower oxidation state analogs but failed in delivering a crucial hydroxy group in the final step. Insight gathered during these studies, however, ultimately led to a synthesis of the pseudoanisatinoids along with the allo-cedrane natural product 11- O-debenzoyltashironin. A second-generation strategy was then developed to access the more highly oxidized majucinoid compounds including jiadifenolide and majucin itself. Overall, one dozen natural products can be accessed from an abundant and inexpensive terpene feedstock. A multitude of general observations regarding site-selective C(sp3)-H bond functionalization reactions in complex polycyclic architectures are reported.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
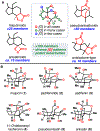
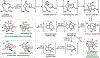
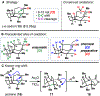


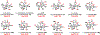
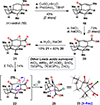


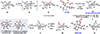
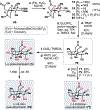
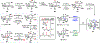
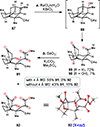

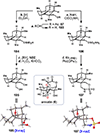
References
-
-
For a general review, see:
Illicium, Pimpinella and Foeniculum; Jodral MM, Ed.; Medicinal and Aromatic Plants – Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2004; Vol. 40.Fukuyama Y; Huang J-M Chemistry and neurotrophic activity of seco-prezizaane- and anislactone-type sesquiterpenes from Illicium species In Bioactive Natural Products (Part L); Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; 2005, Vol. 32, p 395.
-
-
- Lane JF; Koch WT; Leeds NS; Gorin G On the Toxin of Illicium Anisatum. I. The Isolation and Characterization of a Convulsant Principle: Anisatin. J. Am. Chem. Soc 1952, 74, 3211.
- Yamada K; Takada S; Nakamura S; Hirata Y The Structures of Anisatin and Neoanisatin Toxic Sesquiterpenes from Illicium anisatum L. Tetrahedron 1968, 24, 199.
-
- Kouno I; Baba N; Hashimoto M; Kawano N; Takahashi M; Kaneto H; Yang C-S; Sato S Isolation of Three New Sesquiterpene Lactones from the Pericarps of Illicium majus. Chem. Pharm. Bull 1989, 37, 2448.
-
- Kudo Y; Oka J-I; Yamada K Anisatin, a potent GABA antagonist, isolated from Illicium anisatum. Neuroscience Lett. 1981, 25, 83. - PubMed
- Shinozaki H; Ishida M; Kudo Y Effects of anisatin on the GABA action in the crayfish neuromuscular junction. Brain Research 1981, 222, 401. - PubMed
- Ikeda T; Ozoe Y; Okuyama E; Nagata K; Honda H; Shono T; Narahashi T Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br. J. Pharmacol 1999, 127, 1567. - PMC - PubMed
-
- Kuriyama T; Schmidt TJ; Okuyama E; Ozoe Y Structure–Activity Relationships of seco-Prezizaane Terpenoids in γ-Aminobutyric Acid Receptors of Houseflies and Rats. Bioorg. Med. Chem 2002, 10, 1873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

