Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies
- PMID: 30698484
- PMCID: PMC6601548
- DOI: 10.1080/19420862.2019.1574521
Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies
Abstract
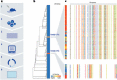
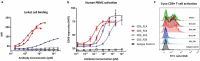
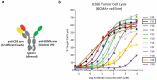
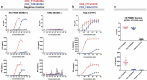
T-cell-recruiting bispecific antibodies (T-BsAbs) have shown potent tumor killing activity in humans, but cytokine release-related toxicities have affected their clinical utility. The use of novel anti-CD3 binding domains with more favorable properties could aid in the creation of T-BsAbs with improved therapeutic windows. Using a sequence-based discovery platform, we identified new anti-CD3 antibodies from humanized rats that bind to multiple epitopes and elicit varying levels of T-cell activation. In T-BsAb format, 12 different anti-CD3 arms induce equivalent levels of tumor cell lysis by primary T-cells, but potency varies by a thousand-fold. Our lead CD3-targeting arm stimulates very low levels of cytokine release, but drives robust tumor antigen-specific killing in vitro and in a mouse xenograft model. This new CD3-targeting antibody underpins a next-generation T-BsAb platform in which potent cytotoxicity is uncoupled from high levels of cytokine release, which may lead to a wider therapeutic window in the clinic.
Keywords: BCMA; Bispecific antibody; CD3; T cell engager; T cells; deep sequencing; multiple myeloma; repertoire.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
