Brain white matter damage and its association with neuronal synchrony during sleep
- PMID: 30698667
- PMCID: PMC6391600
- DOI: 10.1093/brain/awy348
Brain white matter damage and its association with neuronal synchrony during sleep
Abstract
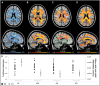
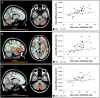
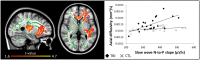
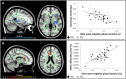
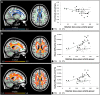
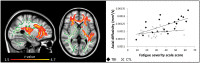
The restorative function of sleep partly relies on its ability to deeply synchronize cerebral networks to create large slow oscillations observable with EEG. However, whether a brain can properly synchronize and produce a restorative sleep when it undergoes massive and widespread white matter damage is unknown. Here, we answer this question by testing 23 patients with various levels of white matter damage secondary to moderate to severe traumatic brain injuries (ages 18-56; 17 males, six females, 11-39 months post-injury) and compared them to 27 healthy subjects of similar age and sex. We used MRI and diffusion tensor imaging metrics (e.g. fractional anisotropy as well as mean, axial and radial diffusivities) to characterize voxel-wise white matter damage. We measured the following slow wave characteristics for all slow waves detected in N2 and N3 sleep stages: peak-to-peak amplitude, negative-to-positive slope, negative and positive phase durations, oscillation frequency, and slow wave density. Correlation analyses were performed in traumatic brain injury and control participants separately, with age as a covariate. Contrary to our hypotheses, we found that greater white matter damage mainly over the frontal and temporal brain regions was strongly correlated with a pattern of higher neuronal synchrony characterized by slow waves of larger amplitudes and steeper negative-to-positive slopes during non-rapid eye movement sleep. The same pattern of associations with white matter damage was also observed with markers of high homeostatic sleep pressure. More specifically, higher white matter damage was associated with higher slow-wave activity power, as well as with more severe complaints of cognitive fatigue. These associations between white matter damage and sleep were found only in our traumatic brain injured participants, with no such correlation in controls. Our results suggest that, contrary to previous observations in healthy controls, white matter damage does not prevent the expected high cerebral synchrony during sleep. Moreover, our observations challenge the current line of hypotheses that white matter microstructure deterioration reduces cerebral synchrony during sleep. Our results showed that the relationship between white matter and the brain's ability to synchronize during sleep is neither linear nor simple.
Keywords: NREM sleep; sleep; traumatic brain injury; white matter.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Epileptic interictal discharges are more frequent during NREM slow wave downstates.Neurosci Lett. 2017 Sep 29;658:37-42. doi: 10.1016/j.neulet.2017.08.020. Epub 2017 Aug 12. Neurosci Lett. 2017. PMID: 28811195
-
Cerebral Gray Matter May Not Explain Sleep Slow-Wave Characteristics after Severe Brain Injury.J Neurosci. 2024 Aug 7;44(32):e1306232024. doi: 10.1523/JNEUROSCI.1306-23.2024. J Neurosci. 2024. PMID: 38844342 Free PMC article.
-
Individual differences in white matter diffusion affect sleep oscillations.J Neurosci. 2013 Jan 2;33(1):227-33. doi: 10.1523/JNEUROSCI.2030-12.2013. J Neurosci. 2013. PMID: 23283336 Free PMC article.
-
Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves.Prog Brain Res. 2011;193:201-18. doi: 10.1016/B978-0-444-53839-0.00013-2. Prog Brain Res. 2011. PMID: 21854964 Free PMC article. Review.
-
Analyzing human sleep EEG: A methodological primer with code implementation.Sleep Med Rev. 2020 Dec;54:101353. doi: 10.1016/j.smrv.2020.101353. Epub 2020 Jul 9. Sleep Med Rev. 2020. PMID: 32736239 Review.
Cited by
-
Integrity of Corpus Callosum Is Essential for theCross-Hemispheric Propagation of Sleep Slow Waves:A High-Density EEG Study in Split-Brain Patients.J Neurosci. 2020 Jul 15;40(29):5589-5603. doi: 10.1523/JNEUROSCI.2571-19.2020. Epub 2020 Jun 15. J Neurosci. 2020. PMID: 32541070 Free PMC article.
-
Track-Weighted Dynamic Functional Connectivity Profiles and Topographic Organization of the Human Pulvinar.Hum Brain Mapp. 2024 Dec 1;45(17):e70062. doi: 10.1002/hbm.70062. Hum Brain Mapp. 2024. PMID: 39639553 Free PMC article.
-
Phase-Amplitude Coupling in Theta and Beta Bands: A Potential Electrophysiological Marker for Obstructive Sleep Apnea.Nat Sci Sleep. 2024 Sep 21;16:1469-1482. doi: 10.2147/NSS.S470617. eCollection 2024. Nat Sci Sleep. 2024. PMID: 39323903 Free PMC article.
-
Sleep spindles are resilient to extensive white matter deterioration.Brain Commun. 2020 Jun 13;2(2):fcaa071. doi: 10.1093/braincomms/fcaa071. eCollection 2020. Brain Commun. 2020. PMID: 32954326 Free PMC article.
-
Enhanced Interplay of Neuronal Coherence and Coupling in the Dying Human Brain.Front Aging Neurosci. 2022 Feb 22;14:813531. doi: 10.3389/fnagi.2022.813531. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35273490 Free PMC article.

