Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis
- PMID: 30698736
- PMCID: PMC6391613
- DOI: 10.1093/brain/awy353
Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis
Abstract
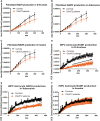
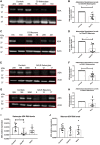
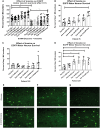
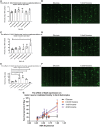
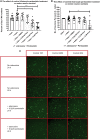
As clinical evidence supports a negative impact of dysfunctional energy metabolism on the disease progression in amyotrophic lateral sclerosis, it is vital to understand how the energy metabolic pathways are altered and whether they can be restored to slow disease progression. Possible approaches include increasing or rerouting catabolism of alternative fuel sources to supplement the glycolytic and mitochondrial pathways such as glycogen, ketone bodies and nucleosides. To analyse the basis of the catabolic defect in amyotrophic lateral sclerosis we used a novel phenotypic metabolic array. We profiled fibroblasts and induced neuronal progenitor-derived human induced astrocytes from C9orf72 amyotrophic lateral sclerosis patients compared to normal controls, measuring the rates of production of reduced nicotinamide adenine dinucleotides from 91 potential energy substrates. This approach shows for the first time that C9orf72 human induced astrocytes and fibroblasts have an adenosine to inosine deamination defect caused by reduction of adenosine deaminase, which is also observed in induced astrocytes from sporadic patients. Patient-derived induced astrocyte lines were more susceptible to adenosine-induced toxicity, which could be mimicked by inhibiting adenosine deaminase in control lines. Furthermore, adenosine deaminase inhibition in control induced astrocytes led to increased motor neuron toxicity in co-cultures, similar to the levels observed with patient derived induced astrocytes. Bypassing metabolically the adenosine deaminase defect by inosine supplementation was beneficial bioenergetically in vitro, increasing glycolytic energy output and leading to an increase in motor neuron survival in co-cultures with induced astrocytes. Inosine supplementation, in combination with modulation of the level of adenosine deaminase may represent a beneficial therapeutic approach to evaluate in patients with amyotrophic lateral sclerosis.
Keywords: C9orf72; ALS; metabolism: inosine: adenosine deaminase.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


Comment in
-
Defects in adenosine metabolism identified in ALS.Nat Rev Neurol. 2019 Mar;15(3):127. doi: 10.1038/s41582-019-0147-7. Nat Rev Neurol. 2019. PMID: 30733617 No abstract available.
References
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 2011; 122: 691–702. - PubMed
-
- Allen CF, Shaw PJ, Ferraiuolo L. Can astrocytes be a target for precision medicine? Adv Exp Med Biol 2017; 1007: 111–28. - PubMed
-
- Allen SP, Duffy LM, Shaw PJ, Grierson AJ. Altered age-related changes in bioenergetic properties and mitochondrial morphology in fibroblasts from sporadic amyotrophic lateral sclerosis patients. Neurobiol Aging 2015; 36: 2893–903. - PubMed
-
- Allen SP, Rajan S, Duffy L, Mortiboys H, Higginbottom A, Grierson AJ, et al. Superoxide dismutase 1 mutation in a cellular model of amyotrophic lateral sclerosis shifts energy generation from oxidative phosphorylation to glycolysis. Neurobiol Aging 2014; 35: 1499–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ALLEN/OCT15/956-799/MNDA_/Motor Neurone Disease Association/United Kingdom
- MR/K01014X/1/MRC_/Medical Research Council/United Kingdom
- SHAW/APR15/933-794/MNDA_/Motor Neurone Disease Association/United Kingdom
- MR/M013251/1/MRC_/Medical Research Council/United Kingdom
- HAUTBERGUE/APR16/846-791/MNDA_/Motor Neurone Disease Association/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

