Intrinsic and regulated properties of minimally edited trypanosome mRNAs
- PMID: 30698753
- PMCID: PMC6468165
- DOI: 10.1093/nar/gkz012
Intrinsic and regulated properties of minimally edited trypanosome mRNAs
Abstract
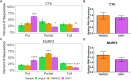
Most mitochondrial mRNAs in kinetoplastids require extensive uridine insertion/deletion editing to generate translatable open reading frames. Editing is specified by trans-acting gRNAs and involves a complex machinery including basal and accessory factors. Here, we utilize high-throughput sequencing to analyze editing progression in two minimally edited mRNAs that provide a simplified system due their requiring only two gRNAs each for complete editing. We show that CYb and MURF2 mRNAs exhibit barriers to editing progression that differ from those previously identified for pan-edited mRNAs, primarily at initial gRNA usage and gRNA exchange. We demonstrate that mis-edited junctions arise through multiple pathways including mis-alignment of cognate gRNA, incorrect and sometimes promiscuous gRNA utilization and inefficient gRNA anchoring. We then examined the roles of accessory factors RBP16 and MRP1/2 in maintaining edited CYb and MURF2 populations. RBP16 is essential for initiation of CYb and MURF2 editing, as well as MURF2 editing progression. In contrast, MRP1/2 stabilizes both edited mRNA populations, while further promoting progression of MURF2 mRNA editing. We also analyzed the effects of RNA Editing Substrate Binding Complex components, TbRGG2 and GAP1, and show that both proteins modestly impact progression of editing on minimally edited mRNAs, suggesting a novel function for GAP1.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures







References
-
- Bilbe G. Overcoming neglect of kinetoplastid diseases. Science. 2015; 348:974–976. - PubMed
-
- D’avila-Levy C.M., Boucinha C., Kostygov A., Santos H.L.C., Morelli K.A., Grybchuk-Ieremenko A., Duval L., Votýpka J., Yurchenko V., Grellier P. et al.. Exploring the environmental diversity of kinetoplastid flagellates in the high-throughput DNA sequencing era. Mem. Inst. Oswaldo Cruz. 2015; 110:956–965. - PMC - PubMed
-
- Jensen R.E., Englund P.T.. Network News: the replication of kinetoplast DNA. Annu. Rev. Microbiol. 2012; 66:473–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

