Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials
- PMID: 30698757
- PMCID: PMC6351779
- DOI: 10.1093/brain/awy319
Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials
Abstract
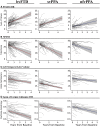
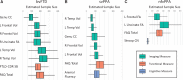
Frontotemporal dementia refers to a group of progressive neurodegenerative syndromes usually caused by the accumulation of pathological tau or TDP-43 proteins. The effects of these proteins in the brain are complex, and each can present with several different clinical syndromes. Clinical efficacy trials of drugs targeting these proteins must use endpoints that are meaningful to all participants despite the variability in symptoms across patients. There are many candidate clinical measures, including neuropsychological scores and functional measures. Brain imaging is another potentially attractive outcome that can be precisely quantified and provides evidence of disease modification. Most imaging studies in frontotemporal dementia have been cross-sectional, and few have compared longitudinal changes in cortical volume with changes in other measures such as perfusion and white matter integrity. The current study characterized longitudinal changes in 161 patients with three frontotemporal dementia syndromes: behavioural variant frontotemporal dementia (n = 77) and the semantic (n = 45) and non-fluent (n = 39) variants of primary progressive aphasia. Visits included comprehensive neuropsychological and functional assessment, structural MRI (3 T), diffusion tensor imaging, and arterial spin labelled perfusion imaging. The goal was to identify measures that are appropriate as clinical trial outcomes for each group, as well as those that might be appropriate for trials that would include more than one of these groups. Linear mixed effects models were used to estimate changes in each measure, and to examine the correlation between imaging and clinical changes. Sample sizes were estimated based on the observed effects for theoretical clinical trials using bootstrapping techniques to provide 95% confidence intervals for these estimates. Declines in functional and neuropsychological measures, as well as frontal and temporal cortical volumes and white matter microstructure were detected in all groups. Imaging changes were statistically significantly correlated with, and explained a substantial portion of variance in, the change in most clinical measures. Perfusion and diffusion tensor imaging accounted for variation in clinical decline beyond volume alone. Sample size estimates for atrophy and diffusion imaging were comparable to clinical measures. Corpus callosal fractional anisotropy led to the lowest sample size estimates for all three syndromes. These findings provide further guidance on selection of trial endpoints for studies in frontotemporal dementia and support the use of neuroimaging, particularly structural and diffusion weighted imaging, as biomarkers. Diffusion and perfusion imaging appear to offer additional utility for explaining clinical change beyond the variance explained by volume alone, arguing for considering multimodal imaging in treatment trials.
Figures