Processing of object motion and self-motion in the lateral subdivision of the medial superior temporal area in macaques
- PMID: 30699042
- PMCID: PMC6485727
- DOI: 10.1152/jn.00497.2018
Processing of object motion and self-motion in the lateral subdivision of the medial superior temporal area in macaques
Abstract
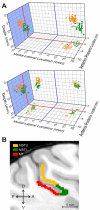
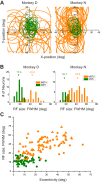
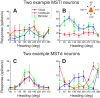
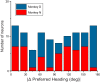
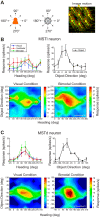
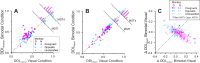
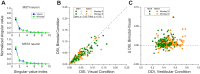
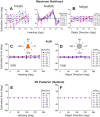
Multiple areas of macaque cortex are involved in visual motion processing, but their relative functional roles remain unclear. The medial superior temporal (MST) area is typically divided into lateral (MSTl) and dorsal (MSTd) subdivisions that are thought to be involved in processing object motion and self-motion, respectively. Whereas MSTd has been studied extensively with regard to processing visual and nonvisual self-motion cues, little is known about self-motion signals in MSTl, especially nonvisual signals. Moreover, little is known about how self-motion and object motion signals interact in MSTl and how this differs from interactions in MSTd. We compared the visual and vestibular heading tuning of neurons in MSTl and MSTd using identical stimuli. Our findings reveal that both visual and vestibular heading signals are weaker in MSTl than in MSTd, suggesting that MSTl is less well suited to participate in self-motion perception than MSTd. We also tested neurons in both areas with a variety of combinations of object motion and self-motion. Our findings reveal that vestibular signals improve the separability of coding of heading and object direction in both areas, albeit more strongly in MSTd due to the greater strength of vestibular signals. Based on a marginalization technique, population decoding reveals that heading and object direction can be more effectively dissociated from MSTd responses than MSTl responses. Our findings help to clarify the respective contributions that MSTl and MSTd make to processing of object motion and self-motion, although our conclusions may be somewhat specific to the multipart moving objects that we employed. NEW & NOTEWORTHY Retinal image motion reflects contributions from both the observer's self-motion and the movement of objects in the environment. The neural mechanisms by which the brain dissociates self-motion and object motion remain unclear. This study provides the first systematic examination of how the lateral subdivision of area MST (MSTl) contributes to dissociating object motion and self-motion. We also examine, for the first time, how MSTl neurons represent translational self-motion based on both vestibular and visual cues.
Keywords: object motion; population code; self-motion; visual cortex.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Bishop CM. Pattern Recognition and Machine Learning. New York: Springer, 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

