Mechanistic framework predicts drug-class specific utility of antiretrovirals for HIV prophylaxis
- PMID: 30699105
- PMCID: PMC6370240
- DOI: 10.1371/journal.pcbi.1006740
Mechanistic framework predicts drug-class specific utility of antiretrovirals for HIV prophylaxis
Abstract
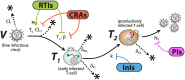
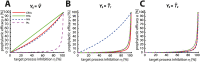
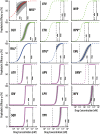
Currently, there is no effective vaccine to halt HIV transmission. However, pre-exposure prophylaxis (PrEP) with the drug combination Truvada can substantially decrease HIV transmission in individuals at risk. Despite its benefits, Truvada-based PrEP is expensive and needs to be taken once-daily, which often leads to inadequate adherence and incomplete protection. These deficits may be overcome by next-generation PrEP regimen, including currently investigated long-acting formulations, or patent-expired drugs. However, poor translatability of animal- and ex vivo/in vitro experiments, and the necessity to conduct long-term (several years) human trials involving considerable sample sizes (N>1000 individuals) are major obstacles to rationalize drug-candidate selection. We developed a prophylaxis modelling tool that mechanistically considers the mode-of-action of all available drugs. We used the tool to screen antivirals for their prophylactic utility and identify lower bound effective concentrations that can guide dose selection in PrEP trials. While in vitro measurable drug potency usually guides PrEP trial design, we found that it may over-predict PrEP potency for all drug classes except reverse transcriptase inhibitors. While most drugs displayed graded concentration-prophylaxis profiles, protease inhibitors tended to switch between none- and complete protection. While several treatment-approved drugs could be ruled out as PrEP candidates based on lack-of-prophylactic efficacy, darunavir, efavirenz, nevirapine, etravirine and rilpivirine could more potently prevent infection than existing PrEP regimen (Truvada). Notably, some drugs from this candidate set are patent-expired and currently neglected for PrEP repurposing. A next step is to further trim this candidate set by ruling out compounds with ominous safety profiles, to assess different administration schemes in silico and to test the remaining candidates in human trials.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Utility of Efavirenz-based Prophylaxis Against HIV Infection. A Systems Pharmacological Analysis.Front Pharmacol. 2019 Mar 13;10:199. doi: 10.3389/fphar.2019.00199. eCollection 2019. Front Pharmacol. 2019. PMID: 30918485 Free PMC article.
-
Modeling HIV Pre-Exposure Prophylaxis.Front Pharmacol. 2020 Jan 31;10:1514. doi: 10.3389/fphar.2019.01514. eCollection 2019. Front Pharmacol. 2020. PMID: 32082142 Free PMC article. Review.
-
Hybrid stochastic framework predicts efficacy of prophylaxis against HIV: An example with different dolutegravir prophylaxis schemes.PLoS Comput Biol. 2018 Jun 14;14(6):e1006155. doi: 10.1371/journal.pcbi.1006155. eCollection 2018 Jun. PLoS Comput Biol. 2018. PMID: 29902179 Free PMC article.
-
Pre-Exposure Prophylaxis for HIV Prevention: Safety Concerns.Drug Saf. 2017 Apr;40(4):273-283. doi: 10.1007/s40264-017-0505-6. Drug Saf. 2017. PMID: 28130774 Free PMC article. Review.
-
The future of pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) infection.Expert Rev Anti Infect Ther. 2017 May;15(5):467-481. doi: 10.1080/14787210.2017.1309292. Epub 2017 Apr 4. Expert Rev Anti Infect Ther. 2017. PMID: 28322067 Review.
Cited by
-
The Utility of Efavirenz-based Prophylaxis Against HIV Infection. A Systems Pharmacological Analysis.Front Pharmacol. 2019 Mar 13;10:199. doi: 10.3389/fphar.2019.00199. eCollection 2019. Front Pharmacol. 2019. PMID: 30918485 Free PMC article.
-
Modeling HIV Pre-Exposure Prophylaxis.Front Pharmacol. 2020 Jan 31;10:1514. doi: 10.3389/fphar.2019.01514. eCollection 2019. Front Pharmacol. 2020. PMID: 32082142 Free PMC article. Review.
-
Modelling the impact of initiation delay, duration and prior PrEP on the efficacy of post-exposure prophylaxis containing a tenofovir/emtricitabine backbone.J Int AIDS Soc. 2025 Jun;28 Suppl 1(Suppl 1):e26454. doi: 10.1002/jia2.26454. J Int AIDS Soc. 2025. PMID: 40569890 Free PMC article.
-
Mathematical modeling to reveal breakthrough mechanisms in the HIV Antibody Mediated Prevention (AMP) trials.PLoS Comput Biol. 2020 Feb 21;16(2):e1007626. doi: 10.1371/journal.pcbi.1007626. eCollection 2020 Feb. PLoS Comput Biol. 2020. PMID: 32084132 Free PMC article.
-
Modeling of HIV-1 prophylactic efficacy and toxicity with islatravir shows non-superiority for oral dosing, but promise as a subcutaneous implant.CPT Pharmacometrics Syst Pharmacol. 2024 Oct;13(10):1693-1706. doi: 10.1002/psp4.13212. Epub 2024 Aug 20. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 39164932 Free PMC article.
References
-
- Grant R, Anderson P, McMahan V, Liu A, Amico K, Mehrotra M, et al. Results of the iPrEx open-label extension (iPrEx OLE) in men and transgender women who have sex with men: PrEP uptake, sexual practices, and HIV incidence. AIDS. 2014; p. 20–25.
-
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. 10.1016/S0140-6736(15)00056-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

