Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface
- PMID: 30699142
- PMCID: PMC6353136
- DOI: 10.1371/journal.pone.0210339
Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface
Abstract
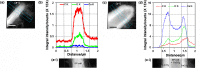
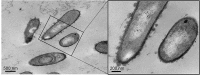
The bacterium Sporosarcina pasteurii (SP) is known for its ability to cause the phenomenon of microbially induced calcium carbonate precipitation (MICP). We explored bacterial participation in the initial stages of the MICP process at the cellular length scale under two different growth environments (a) liquid culture (b) MICP in a soft agar (0.5%) column. In the liquid culture, ex-situ imaging of the cellular environment indicated that S. pasteurii was facilitating nucleation of nanoscale crystals of calcium carbonate on bacterial cell surface and its growth via ureolysis. During the same period, the meso-scale environment (bulk medium) was found to have overgrown calcium carbonate crystals. The effect of media components (urea, CaCl2), presence of live and dead in the growth medium were explored. The agar column method allows for in-situ visualization of the phenomena, and using this platform, we found conclusive evidence of the bacterial cell surface facilitating formation of nanoscale crystals in the microenvironment. Here also the bulk environment or the meso-scale environment was found to possess overgrown calcium carbonate crystals. Extensive elemental analysis using Energy dispersive X-ray spectroscopy (EDS) and X-ray powder diffraction (XRD), confirmed that the crystals to be calcium carbonate, and two different polymorphs (calcite and vaterite) were identified. Active participation of S. pasteurii cell surface as the site of calcium carbonate precipitation has been shown using EDS elemental mapping with Scanning transmission electron microscopy (STEM) and scanning electron microscopy (SEM).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Stocks-Fischer S, Galinat JK, Bang SS. Microbiological precipitation of CaCO 3. Soil Biology and Biochemistry. 1999;31(11):1563–1571.
-
- Warren LA, Maurice PA, Parmar N, Ferris FG. Microbially mediated calcium carbonate precipitation: implications for interpreting calcite precipitation and for solid-phase capture of inorganic contaminants. Geomicrobiology Journal. 2001;18(1):93–115.
-
- De Muynck W, De Belie N, Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecological Engineering. 2010;36(2):118–136.
-
- Thompson J, Ferris F. Cyanobacterial precipitation of gypsum, calcite, and magnesite from natural alkaline lake water. Geology. 1990;18(10):995–998.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

