A Placebo-Controlled, Randomized Trial of Enarodustat in Patients with Chronic Kidney Disease Followed by Long-Term Trial
- PMID: 30699415
- PMCID: PMC6481254
- DOI: 10.1159/000496929
A Placebo-Controlled, Randomized Trial of Enarodustat in Patients with Chronic Kidney Disease Followed by Long-Term Trial
Abstract
Background: Enarodustat (JTZ-951) is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that mimics adaptive responses to hypoxic conditions and may provide a new therapeutic approach for managing anemia in patients with chronic kidney disease (CKD). We evaluated the efficacy, safety, and maintenance dose of enarodustat in anemic patients with CKD not on dialysis.
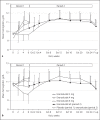
Methods: Erythropoiesis-stimulating agent (ESA) naïve patients (correction group) and patients on a stable dose of ESA (conversion group) were randomized to receive 2, 4, or 6 mg of enarodustat or placebo once daily for 6 weeks in a double-blind manner (Period 1) followed by 24 weeks of open enarodustat treatment to maintain their hemoglobin (Hb) levels within a target range of 10.0-12.0 g/dL in reference to a dose adjustment algorithm (Period 2).
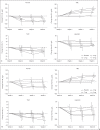
Results: In the correction group, Hb level increase rate per week increased in a dose-response manner. The proportion of subjects in the conversion group who maintained Hb levels within ± 1.0 g/dL of baseline did not differ between each enarodustat arm and placebo arm during Period 1. Over 70% of subjects in both groups maintained Hb levels within the target range at the end of treatment in Period 2. The mean prescribed doses were 3.58 and 3.74 mg/day in the correction group and the conversion group, respectively. Enarodustat was associated with decreases in hepcidin and ferritin and increased total iron-binding capacity and was generally well tolerated.
Conclusions: Enarodustat corrects and maintains Hb levels in anemic patients with CKD not on dialysis.
Keywords: Anemia in chronic kidney disease; Enarodustat; Hepcidin; Hypoxia-inducible factor prolyl hydroxylase inhibitor; Randomized trial.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Figures
References
-
- Artunc F, Risler T. Serum erythropoietin concentrations and responses to anaemia in patients with or without chronic kidney disease. Nephrol Dial Transplant. 2007;22:2900–2908. - PubMed
-
- Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. Am J Kidney Dis. 2011;58:591–598. - PubMed
-
- Scrutinio D, Passantino A, Santoro D, Catanzaro R. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–67. - PubMed
-
- Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

