New Chloramphenicol Derivatives from the Viewpoint of Anticancer and Antimicrobial Activity
- PMID: 30699905
- PMCID: PMC6466596
- DOI: 10.3390/antibiotics8010009
New Chloramphenicol Derivatives from the Viewpoint of Anticancer and Antimicrobial Activity
Abstract
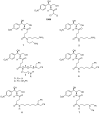
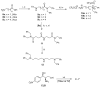
Over the last years, we have been focused on chloramphenicol conjugates that combine in their structure chloramphenicol base with natural polyamines, spermine, spermidine and putrescine, and their modifications. Conjugate 3, with spermidine (SPD) as a natural polyamine linked to chloramphenicol base, showed the best antibacterial and anticancer properties. Using 3 as a prototype, we here explored the influence of the antibacterial and anticancer activity of additional benzyl groups on N1 amino moiety together with modifications of the alkyl length of the aminobutyl fragment of SPD. Our data demonstrate that the novel modifications did not further improve the antibacterial activity of the prototype. However, one of the novel conjugates (4) showed anticancer activity without affecting bacterial growth, thus emerging as a promising anticancer agent, with no adverse effects on bacterial microflora when taken orally.
Keywords: Chloramphenicol; antibiotics; anticancer activity; antimicrobial activity; conjugates; mitochondrial ribosome; molecular dynamics simulations; polyamines; protein biosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells.Cancer Res. 1985 May;45(5):2050-7. Cancer Res. 1985. PMID: 3921235
-
Synthesis and antimicrobial activity of chloramphenicol-polyamine conjugates.Bioorg Med Chem. 2015 Jul 1;23(13):3163-74. doi: 10.1016/j.bmc.2015.04.069. Epub 2015 May 1. Bioorg Med Chem. 2015. PMID: 26001343
-
Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth.Cancer Res. 1987 Jun 1;47(11):2821-5. Cancer Res. 1987. PMID: 3567905
-
Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 2. Structural analogues and derivatives.Curr Drug Targets. 2003 Oct;4(7):565-85. doi: 10.2174/1389450033490876. Curr Drug Targets. 2003. PMID: 14535655 Review.
-
Catabolism of polyamines.Amino Acids. 2004 Jun;26(3):217-33. doi: 10.1007/s00726-004-0070-z. Epub 2004 Apr 20. Amino Acids. 2004. PMID: 15221502 Review.
Cited by
-
Conjugates of Chloramphenicol Amine and Berberine as Antimicrobial Agents.Antibiotics (Basel). 2022 Dec 22;12(1):15. doi: 10.3390/antibiotics12010015. Antibiotics (Basel). 2022. PMID: 36671216 Free PMC article.
-
Unveiling synergism of polymyxin B with chloramphenicol derivatives against multidrug-resistant (MDR) Klebsiella pneumoniae.J Antibiot (Tokyo). 2023 Dec;76(12):711-719. doi: 10.1038/s41429-023-00659-2. Epub 2023 Oct 11. J Antibiot (Tokyo). 2023. PMID: 37821539
-
Recent Trends in Synthesis of Chloramphenicol New Derivatives.Antibiotics (Basel). 2021 Mar 31;10(4):370. doi: 10.3390/antibiotics10040370. Antibiotics (Basel). 2021. PMID: 33807439 Free PMC article.
-
Broad-Spectrum Antibiotic Use and Disease Progression in Early-Stage Melanoma Patients: A Retrospective Cohort Study.Cancers (Basel). 2021 Aug 29;13(17):4367. doi: 10.3390/cancers13174367. Cancers (Basel). 2021. PMID: 34503177 Free PMC article.
-
Triphenilphosphonium Analogs of Chloramphenicol as Dual-Acting Antimicrobial and Antiproliferating Agents.Antibiotics (Basel). 2021 Apr 23;10(5):489. doi: 10.3390/antibiotics10050489. Antibiotics (Basel). 2021. PMID: 33922611 Free PMC article.
References
-
- Dinos G., Athanassopoulos C., Missiri D., Giannopoulou P., Vlachogiannis I., Papadopoulos G., Papaioannou D., Kalpaxis D. Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics. 2016;5:20. doi: 10.3390/antibiotics5020020. - DOI - PMC - PubMed
-
- Lamb R., Ozsvari B., Lisanti C.L., Tanowitz H.B., Howell A., Martinez-Outschoorn U.E., Sotgia F., Lisanti M.P. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget. 2015;6:4569–4584. doi: 10.18632/oncotarget.3174. - DOI - PMC - PubMed
-
- Marks J., Kannan K., Roncase E.J., Klepacki D., Kefi A., Orelle C., Vázquez-Laslop N., Mankin A.S. Context-specific inhibition of translation by ribosomal antibiotics targeting the peptidyl transferase center. Proc. Natl. Acad. Sci. USA. 2016;113:12150–12155. doi: 10.1073/pnas.1613055113. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

