Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO₂ under Various Atmospheres
- PMID: 30699943
- PMCID: PMC6409930
- DOI: 10.3390/nano9020163
Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO₂ under Various Atmospheres
Abstract
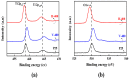
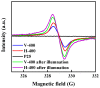
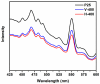
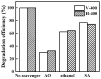
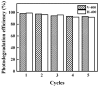
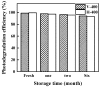
In this report, the photocatalytic activity of P25 has been explored and the influence of thermal treatment under various atmospheres (air, vacuum and hydrogen) were discussed. The samples' characteristics were disclosed by means of various instruments including X-ray diffraction (XRD), Electron paramagnetic resonance (EPR), X-ray photoelectron spectroscopy (XPS) and UV⁻vis. This study also accentuates various states of the oxygen vacancy density formed inside the samples as well as the colour turning observed in treated P25 under various atmospheres. Produced coloured TiO₂ samples were then exploited for their photocatalytic capability concerning photodegradation of methylene blue (MB) using air mass (AM) 1.5 G solar light irradiation. Our findings revealed that exceptional photocatalytic activity of P25 is related to the thermal treatment. Neither oxygen vacancy formation nor photocatalytic activity enhancement was observed in the air-treated sample. H₂-treated samples have shown better photoactivity which even could be further improved by optimizing treatment conditions to achieve the advantages of the positive role of oxygen vacancy (O-vacancy at higher concentration than optimum acts as electron trapping sites). The chemical structure and stability of the samples were also studied. There was no sign of deteriorating of O₂-vacancies inside the samples after 6 months. High stability of thermal treated samples in terms of both long and short-term time intervals is another significant feature of the produced photocatalyst.
Keywords: degradation; hydrogen; oxygen vacancy; thermal treatment; vacuum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis.ACS Appl Mater Interfaces. 2018 Oct 17;10(41):35316-35326. doi: 10.1021/acsami.8b14680. Epub 2018 Oct 2. ACS Appl Mater Interfaces. 2018. PMID: 30226370
-
Enhanced Photocatalytic Activity of Vacuum-activated TiO2 Induced by Oxygen Vacancies.Photochem Photobiol. 2018 May;94(3):472-483. doi: 10.1111/php.12874. Epub 2018 Mar 2. Photochem Photobiol. 2018. PMID: 29253309
-
Facile synthesis of thermal- and photostable titania with paramagnetic oxygen vacancies for visible-light photocatalysis.Chemistry. 2013 Feb 18;19(8):2866-73. doi: 10.1002/chem.201202833. Epub 2013 Jan 10. Chemistry. 2013. PMID: 23307339
-
Green synthetic approach for Ti3+ self-doped TiO(2-x) nanoparticles with efficient visible light photocatalytic activity.Nanoscale. 2013 Mar 7;5(5):1870-5. doi: 10.1039/c2nr33563h. Epub 2013 Jan 24. Nanoscale. 2013. PMID: 23348572
-
One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity.Nanoscale. 2012 Jan 21;4(2):576-84. doi: 10.1039/c1nr11353d. Epub 2011 Dec 5. Nanoscale. 2012. PMID: 22143193
Cited by
-
Visible Light Driven Heterojunction Photocatalyst of CuO-Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants.Nanomaterials (Basel). 2019 Jul 13;9(7):1011. doi: 10.3390/nano9071011. Nanomaterials (Basel). 2019. PMID: 31337085 Free PMC article.
-
Nanoengineered Advanced Materials for Enabling Hydrogen Economy: Functionalized Graphene-Incorporated Cupric Oxide Catalyst for Efficient Solar Hydrogen Production.Glob Chall. 2020 Jan 24;4(3):1900087. doi: 10.1002/gch2.201900087. eCollection 2020 Mar. Glob Chall. 2020. PMID: 32140256 Free PMC article.
-
Hollow anatase TiO2 tetrakaidecahedral crystals with an active {001}/{110} redox interface toward high-performance photocatalytic activity.Chem Sci. 2023 Dec 5;15(2):692-700. doi: 10.1039/d3sc04328b. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179522 Free PMC article.
References
-
- Nakata K., Fujishima A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. 2012;13:169–189. doi: 10.1016/j.jphotochemrev.2012.06.001. - DOI
-
- Gonell F., Puga A.V., López B.J., García H., Corma A. Copper-doped titania photocatalysts for simultaneous reduction of CO2 and production of H2 from aqueous sulfide. Appl. Catal. B Environ. 2016;180:263–270. doi: 10.1016/j.apcatb.2015.06.019. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

