Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice
- PMID: 30699944
- PMCID: PMC6387212
- DOI: 10.3390/ijms20030568
Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice
Abstract
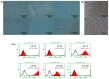
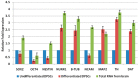
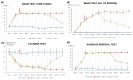
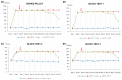
Parkinson's disease (PD) is a neurodegenerative disorder defined by progressive deterioration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Dental pulp stem cells (DPSCs) have been proposed to replace the degenerated dopaminergic neurons due to its inherent neurogenic and regenerative potential. However, the effective delivery and homing of DPSCs within the lesioned brain has been one of the many obstacles faced in cell-based therapy of neurodegenerative disorders. We hypothesized that DPSCs, delivered intranasally, could circumvent these challenges. In the present study, we investigated the therapeutic efficacy of intranasally administered DPSCs in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of PD. Human deciduous DPSCs were cultured, pre-labelled with PKH 26, and intranasally delivered into PD mice following MPTP treatment. Behavioural analyses were performed to measure olfactory function and sensorimotor coordination, while tyrosine hydroxylase (TH) immunofluorescence was used to evaluate MPTP neurotoxicity in SNpc neurons. Upon intranasal delivery, degenerated TH-positive neurons were ameliorated, while deterioration in behavioural performances was significantly enhanced. Thus, the intranasal approach enriched cell delivery to the brain, optimizing its therapeutic potential through its efficacious delivery and protection against dopaminergic neuron degeneration.
Keywords: MPTP; Parkinson’s disease; behavioural analysis; dental pulp stem cells; intranasal delivery; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effect of dental pulp stem cells in MPTP-induced old-aged mice model.Eur J Clin Invest. 2017 Jun;47(6):403-414. doi: 10.1111/eci.12753. Epub 2017 Apr 27. Eur J Clin Invest. 2017. PMID: 28369799
-
c-Jun/Bim Upregulation in Dopaminergic Neurons Promotes Neurodegeneration in the MPTP Mouse Model of Parkinson's Disease.Neuroscience. 2019 Feb 10;399:117-124. doi: 10.1016/j.neuroscience.2018.12.026. Epub 2018 Dec 25. Neuroscience. 2019. PMID: 30590105
-
Assessment of the Effects of MPTP and Paraquat on Dopaminergic Neurons and Microglia in the Substantia Nigra Pars Compacta of C57BL/6 Mice.PLoS One. 2016 Oct 27;11(10):e0164094. doi: 10.1371/journal.pone.0164094. eCollection 2016. PLoS One. 2016. PMID: 27788145 Free PMC article.
-
COX-2 and neurodegeneration in Parkinson's disease.Ann N Y Acad Sci. 2003 Jun;991:272-7. doi: 10.1111/j.1749-6632.2003.tb07482.x. Ann N Y Acad Sci. 2003. PMID: 12846993 Review.
-
The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson's disease.Stem Cell Res Ther. 2021 Jan 6;12(1):5. doi: 10.1186/s13287-020-01957-4. Stem Cell Res Ther. 2021. PMID: 33407864 Free PMC article. Review.
Cited by
-
The potential roles of dental pulp stem cells in peripheral nerve regeneration.Front Neurol. 2023 Jan 11;13:1098857. doi: 10.3389/fneur.2022.1098857. eCollection 2022. Front Neurol. 2023. PMID: 36712432 Free PMC article. Review.
-
The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies.Cells. 2023 Jun 22;12(13):1686. doi: 10.3390/cells12131686. Cells. 2023. PMID: 37443720 Free PMC article. Review.
-
Intranasal Transplantation of Microbiota Derived from Parkinson's Disease Mice Induced Astrocyte Activation and Neurodegenerative Pathology from Nose to Brain.Brain Sci. 2025 Apr 23;15(5):433. doi: 10.3390/brainsci15050433. Brain Sci. 2025. PMID: 40426604 Free PMC article.
-
Nose-to-Brain: The Next Step for Stem Cell and Biomaterial Therapy in Neurological Disorders.Cells. 2022 Oct 1;11(19):3095. doi: 10.3390/cells11193095. Cells. 2022. PMID: 36231058 Free PMC article. Review.
-
Advances in intranasal application of stem cells in the treatment of central nervous system diseases.Stem Cell Res Ther. 2021 Mar 24;12(1):210. doi: 10.1186/s13287-021-02274-0. Stem Cell Res Ther. 2021. PMID: 33762014 Free PMC article. Review.
References
-
- Perry E.K., Mckeith I., Thompson P., Marshall E., Kerwin J., Jabeen S., Edwardson J.A., Ince P., Blessed G., Irving D., et al. Topography, Extent, and Clinical Relevance of Neurochemical Deficits in Dementia of Lewy Body Type, Parkinson’s Disease, and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1991;640:197–202. doi: 10.1111/j.1749-6632.1991.tb00217.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

