4- O-Methylhonokiol Influences Normal Cardiovascular Development in Medaka Embryo
- PMID: 30699965
- PMCID: PMC6384692
- DOI: 10.3390/molecules24030475
4- O-Methylhonokiol Influences Normal Cardiovascular Development in Medaka Embryo
Abstract
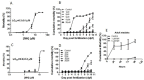
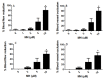
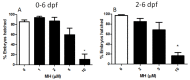
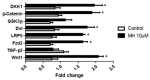
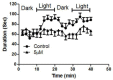
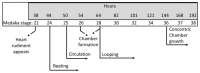
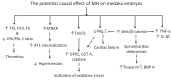
Although 4-O-Methylhonokiol (MH) effects on neuronal and immune cells have been established, it is still unclear whether MH can cause a change in the structure and function of the cardiovascular system. The overarching goal of this study was to evaluate the effects of MH, isolated from Magnolia grandiflora, on the development of the heart and vasculature in a Japanese medaka model in vivo to predict human health risks. We analyzed the toxicity of MH in different life-stages of medaka embryos. MH uptake into medaka embryos was quantified. The LC50 of two different exposure windows (stages 9⁻36 (0⁻6 days post fertilization (dpf)) and 25⁻36 (2⁻6 dpf)) were 5.3 ± 0.1 μM and 9.9 ± 0.2 μM. Survival, deformities, days to hatch, and larval locomotor response were quantified. Wnt 1 was overexpressed in MH-treated embryos indicating deregulation of the Wnt signaling pathway, which was associated with spinal and cardiac ventricle deformities. Overexpression of major proinflammatory mediators and biomarkers of the heart were detected. Our results indicated that the differential sensitivity of MH in the embryos was developmental stage-specific. Furthermore, this study demonstrated that certain molecules can serve as promising markers at the transcriptional and phenotypical levels, responding to absorption of MH in the developing embryo.
Keywords: cardiomyogenesis; factor VII; factor X; herbal medicine; inflammation; thrombosis; vasculogenesis.
Conflict of interest statement
The authors declare no competing interests. The contents of this article are the sole responsibility of the authors and do not necessarily represent the official University of Mississippi or the grant agency officials.
Figures



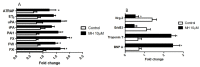

Similar articles
-
Magnolia bioactive constituent 4-O-methylhonokiol prevents the impairment of cardiac insulin signaling and the cardiac pathogenesis in high-fat diet-induced obese mice.Int J Biol Sci. 2015 Jun 5;11(8):879-91. doi: 10.7150/ijbs.12101. eCollection 2015. Int J Biol Sci. 2015. PMID: 26157343 Free PMC article.
-
RANKL-induced osteoclastogenesis is suppressed by 4-O-methylhonokiol in bone marrow-derived macrophages.Arch Pharm Res. 2017 Aug;40(8):933-942. doi: 10.1007/s12272-017-0932-z. Epub 2017 Jul 24. Arch Pharm Res. 2017. PMID: 28736799
-
Alteration of development and gene expression induced by in ovo-nanoinjection of 3-hydroxybenzo[c]phenanthrene into Japanese medaka (Oryzias latipes) embryos.Aquat Toxicol. 2017 Jan;182:194-204. doi: 10.1016/j.aquatox.2016.11.020. Epub 2016 Nov 25. Aquat Toxicol. 2017. PMID: 27930992
-
Japanese medaka (Oryzias latipes): developmental model for the study of alcohol teratology.Birth Defects Res B Dev Reprod Toxicol. 2006 Feb;77(1):29-39. doi: 10.1002/bdrb.20072. Birth Defects Res B Dev Reprod Toxicol. 2006. PMID: 16496295
-
[Advance in latest studies on pharmacological effects of magnolol].Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3526-30. Zhongguo Zhong Yao Za Zhi. 2012. PMID: 23477132 Review. Chinese.
Cited by
-
In Vitro and In Silico Studies of Neolignans from Magnolia grandiflora L. Seeds against Human Cannabinoids and Opioid Receptors.Molecules. 2023 Jan 27;28(3):1253. doi: 10.3390/molecules28031253. Molecules. 2023. PMID: 36770918 Free PMC article.
-
Histopathology of the Intervertebral Disc of Nothobranchius furzeri, a Fish Model of Accelerated Aging.Biology (Basel). 2023 Oct 3;12(10):1305. doi: 10.3390/biology12101305. Biology (Basel). 2023. PMID: 37887015 Free PMC article.
References
-
- Dominguez F., Chavez M., Garduno-Ramirez M.L., Chavez-Avila V.M., Mata M., Cruz-Sosa F. Honokiol and magnolol production by in vitro micropropagated plants of Magnolia dealbata, an endangered endemic Mexican species. Nat. Prod. Commun. 2010;5:235–240. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

