The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence
- PMID: 30699983
- PMCID: PMC6387184
- DOI: 10.3390/ijms20030575
The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence
Abstract
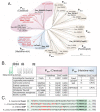
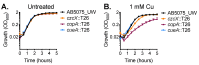
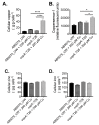
Acinetobacter baumannii has emerged as one of the leading causative agents of nosocomial infections. Due to its high level of intrinsic and adapted antibiotic resistance, treatment failure rates are high, which allows this opportunistic pathogen to thrive during infection in immune-compromised patients. A. baumannii can cause infections within a broad range of host niches, with pneumonia and bacteraemia being associated with the greatest levels of morbidity and mortality. Although its resistance to antibiotics is widely studied, our understanding of the mechanisms required for dealing with environmental stresses related to virulence and hospital persistence, such as copper toxicity, is limited. Here, we performed an in silico analysis of the A. baumannii copper resistome, examining its regulation under copper stress. Using comparative analyses of bacterial P-type ATPases, we propose that A. baumannii encodes a member of a novel subgroup of P1B-1 ATPases. Analyses of three putative inner membrane copper efflux systems identified the P1B-1 ATPase CopA as the primary mediator of cytoplasmic copper resistance in A. baumannii. Using a murine model of A. baumannii pneumonia, we reveal that CopA contributes to the virulence of A. baumannii. Collectively, this study advances our understanding of how A. baumannii deals with environmental copper toxicity, and it provides novel insights into how A. baumannii combats adversities encountered as part of the host immune defence.
Keywords: P-type ATPases; P1B-1; bacterial; metal ions; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . WHO Publishes List of Bacteria for which New Antibiotics Are Urgently Needed. WHO Media Centre; Geneva, Switzerland: 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

