Potential Role of H-Ferritin in Mitigating Valvular Mineralization
- PMID: 30700131
- PMCID: PMC6393195
- DOI: 10.1161/ATVBAHA.118.312191
Potential Role of H-Ferritin in Mitigating Valvular Mineralization
Abstract
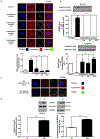
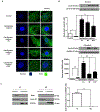
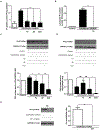
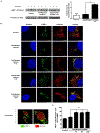
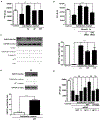
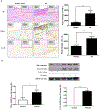
Objective- Calcific aortic valve disease is a prominent finding in elderly and in patients with chronic kidney disease. We investigated the potential role of iron metabolism in the pathogenesis of calcific aortic valve disease. Approach and Results- Cultured valvular interstitial cells of stenotic aortic valve with calcification from patients undergoing valve replacement exhibited significant susceptibility to mineralization/osteoblastic transdifferentiation in response to phosphate. This process was abrogated by iron via induction of H-ferritin as reflected by lowering ALP and osteocalcin secretion and preventing extracellular calcium deposition. Cellular phosphate uptake and accumulation of lysosomal phosphate were decreased. Accordingly, expression of phosphate transporters Pit1 and Pit2 were repressed. Translocation of ferritin into lysosomes occurred with high phosphate-binding capacity. Importantly, ferritin reduced nuclear accumulation of RUNX2 (Runt-related transcription factor 2), and as a reciprocal effect, it enhanced nuclear localization of transcription factor Sox9 (SRY [sex-determining region Y]-box 9). Pyrophosphate generation was also increased via upregulation of ENPP2 (ectonucleotide pyrophosphatase/phosphodiesterase-2). 3H-1, 2-dithiole-3-thione mimicked these beneficial effects in valvular interstitial cell via induction of H-ferritin. Ferroxidase activity of H-ferritin was essential for this function, as ceruloplasmin exhibited similar inhibitory functions. Histological analysis of stenotic aortic valve revealed high expression of H-ferritin without iron accumulation and its relative dominance over ALP in noncalcified regions. Increased expression of H-ferritin accompanied by elevation of TNF-α (tumor necrosis factor-α) and IL-1β (interleukin-1β) levels, inducers of H-ferritin, corroborates the essential role of ferritin/ferroxidase via attenuating inflammation in calcific aortic valve disease. Conclusions- Our results indicate that H-ferritin is a stratagem in mitigating valvular mineralization/osteoblastic differentiation. Utilization of 3H-1, 2-dithiole-3-thione to induce ferritin expression may prove a novel therapeutic potential in valvular mineralization.
Keywords: arteriosclerosis; chronic kidney disease; phosphate; stenosis; vascular calcification.
Conflict of interest statement
Disclosures
All the authors declared no competing interests.
Figures




References
-
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011; 124: 1783–1791. - PMC - PubMed
-
- Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 2001; 103: 1522–1528. - PubMed
-
- Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, Tanganelli P. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. Journal of the American College of Cardiology 2004; 43: 1670–1676. - PubMed
-
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111: 3316–3326. - PubMed
-
- Dweck MR, Boon NA, Newby DE. Calcific Aortic Stenosis: A Disease of the Valve and the Myocardium. Journal of the American College of Cardiology 2012; 60: 1854–1863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

