Evolution of vertebrate nicotinic acetylcholine receptors
- PMID: 30700248
- PMCID: PMC6354393
- DOI: 10.1186/s12862-018-1341-8
Evolution of vertebrate nicotinic acetylcholine receptors
Abstract
Background: Many physiological processes are influenced by nicotinic acetylcholine receptors (nAChR), ranging from neuromuscular and parasympathetic signaling to modulation of the reward system and long-term memory. Due to the complexity of the nAChR family and variable evolutionary rates among its members, their evolution in vertebrates has been difficult to resolve. In order to understand how and when the nAChR genes arose, we have used a broad approach of analyses combining sequence-based phylogeny, chromosomal synteny and intron positions.
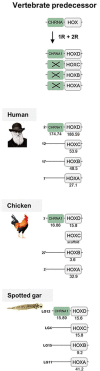
Results: Our analyses suggest that there were ten subunit genes present in the vertebrate predecessor. The two basal vertebrate tetraploidizations (1R and 2R) then expanded this set to 19 genes. Three of these have been lost in mammals, resulting in 16 members today. None of the ten ancestral genes have kept all four copies after 2R. Following 2R, two of the ancestral genes became triplicates, five of them became pairs, and three seem to have remained single genes. One triplet consists of CHRNA7, CHRNA8 and the previously undescribed CHRNA11, of which the two latter have been lost in mammals but are still present in lizards and ray-finned fishes. The other triplet consists of CHRNB2, CHRNB4 and CHRNB5, the latter of which has also been lost in mammals. In ray-finned fish the neuromuscular subunit gene CHRNB1 underwent a local gene duplication generating CHRNB1.2. The third tetraploidization in the predecessor of teleosts (3R) expanded the repertoire to a total of 31 genes, of which 27 remain in zebrafish. These evolutionary relationships are supported by the exon-intron organization of the genes.
Conclusion: The tetraploidizations explain all gene duplication events in vertebrates except two. This indicates that the genome doublings have had a substantial impact on the complexity of this gene family leading to a very large number of members that have existed for hundreds of millions of years.
Keywords: Acetylcholine; Gene duplication; Nicotinic; Ohnolog; Paralogon; Receptor; Spotted gar; Synteny; Tetraploidization; Zebrafish.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures








Similar articles
-
New Insights Into the Evolutionary History of Melatonin Receptors in Vertebrates, With Particular Focus on Teleosts.Front Endocrinol (Lausanne). 2020 Sep 24;11:538196. doi: 10.3389/fendo.2020.538196. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33071966 Free PMC article. Review.
-
The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications.BMC Evol Biol. 2013 Nov 2;13:238. doi: 10.1186/1471-2148-13-238. BMC Evol Biol. 2013. PMID: 24180662 Free PMC article.
-
Corticotropin-releasing hormone family evolution: five ancestral genes remain in some lineages.J Mol Endocrinol. 2016 Jul;57(1):73-86. doi: 10.1530/JME-16-0051. Epub 2016 May 24. J Mol Endocrinol. 2016. PMID: 27220618
-
Evolution of the Muscarinic Acetylcholine Receptors in Vertebrates.eNeuro. 2018 Nov 8;5(5):ENEURO.0340-18.2018. doi: 10.1523/ENEURO.0340-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30564629 Free PMC article.
-
New insights into the evolution of vertebrate CRH (corticotropin-releasing hormone) and invertebrate DH44 (diuretic hormone 44) receptors in metazoans.Gen Comp Endocrinol. 2014 Dec 1;209:162-70. doi: 10.1016/j.ygcen.2014.09.004. Epub 2014 Sep 16. Gen Comp Endocrinol. 2014. PMID: 25230393 Review.
Cited by
-
CHRNB2 represses pancreatic cancer migration and invasion via inhibiting β-catenin pathway.Cancer Cell Int. 2022 Nov 7;22(1):340. doi: 10.1186/s12935-022-02768-8. Cancer Cell Int. 2022. PMID: 36344976 Free PMC article.
-
Characterization of nAChRs in Nematostella vectensis supports neuronal and non-neuronal roles in the cnidarian-bilaterian common ancestor.Evodevo. 2019 Nov 2;10:27. doi: 10.1186/s13227-019-0136-3. eCollection 2019. Evodevo. 2019. PMID: 31700598 Free PMC article.
-
The Structure, Function, and Physiology of the Fetal and Adult Acetylcholine Receptor in Muscle.Front Mol Neurosci. 2020 Sep 8;13:581097. doi: 10.3389/fnmol.2020.581097. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33013323 Free PMC article. Review.
-
Identification of slit3 as a locus affecting nicotine preference in zebrafish and human smoking behaviour.Elife. 2020 Mar 25;9:e51295. doi: 10.7554/eLife.51295. Elife. 2020. PMID: 32209227 Free PMC article.
-
DMPP attenuates lipopolysaccharide-induced lung injury by inhibiting glycocalyx degradation through activation of the cholinergic anti-inflammatory pathway.J Bioenerg Biomembr. 2023 Dec;55(6):447-456. doi: 10.1007/s10863-023-09989-0. Epub 2023 Oct 18. J Bioenerg Biomembr. 2023. PMID: 37851169
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

